Development and Assessment of Herpes Simplex Virus Type 1 (HSV-1) Amplicon Vectors with Sensory Neuron-Selective Promoters
- PMID: 35955608
- PMCID: PMC9369297
- DOI: 10.3390/ijms23158474
Development and Assessment of Herpes Simplex Virus Type 1 (HSV-1) Amplicon Vectors with Sensory Neuron-Selective Promoters
Abstract
Background: Neurogenic detrusor overactivity (NDO) is a severe pathological condition characterized by involuntary detrusor contractions leading to urine leakage. This condition is frequent after spinal cord injury (SCI). Gene therapy for NDO requires the development of vectors that express therapeutic transgenes driven by sensory neuron-specific promoters. The aim of this study was to develop and assess tools for the characterization of sensory neuron-specific promoters in dorsal root ganglia (DRG) neurons after transduction with herpes simplex virus type 1 (HSV-1)-based amplicon defective vectors.
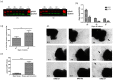
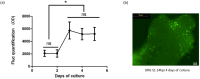
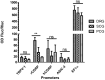
Methods: The HSV-1 vector genome encoded two independent transcription cassettes: one expressed firefly luciferase (FLuc) driven by different promoters' candidates (rTRPV1, rASIC3, rCGRP, or hCGRP), and the other expressed a reporter gene driven by an invariable promoter. The strength and selectivity of promoters was assessed in organotypic cultures of explanted adult DRG, or sympathetic and parasympathetic ganglia from control and SCI rats.
Results: The rCGRP promoter induced selective expression in the DRG of normal rats. The rTRPV-1 promoter, which did not display selective activity in control rats, induced selective expression in DRG explanted from SCI rats.
Conclusions: This study provides a methodology to assess sensory neuron-specific promoters, opening new perspectives for future gene therapy for NDO.
Keywords: HSV-1 amplicon vectors; organotypic cultures; peripheral ganglia; sensory neurons; specific promoter.
Conflict of interest statement
C.J., F.G. and A.L.E. are co-founders of EG 427. A.L.E. is working for EG 427.
Figures


Similar articles
-
Non-replicative herpes simplex virus genomic and amplicon vectors for gene therapy - an update.Gene Ther. 2025 May;32(3):173-183. doi: 10.1038/s41434-024-00500-x. Epub 2024 Nov 12. Gene Ther. 2025. PMID: 39533042 Free PMC article. Review.
-
Morphological changes in different populations of bladder afferent neurons detected by herpes simplex virus (HSV) vectors with cell-type-specific promoters in mice with spinal cord injury.Neuroscience. 2017 Nov 19;364:190-201. doi: 10.1016/j.neuroscience.2017.09.024. Epub 2017 Sep 20. Neuroscience. 2017. PMID: 28942324 Free PMC article.
-
Herpes simplex virus vector-mediated gene delivery of glutamic acid decarboxylase reduces detrusor overactivity in spinal cord-injured rats.Gene Ther. 2009 May;16(5):660-8. doi: 10.1038/gt.2009.5. Epub 2009 Feb 19. Gene Ther. 2009. PMID: 19225548 Free PMC article.
-
Botulinum Neurotoxin Light Chains Expressed by Defective Herpes Simplex Virus Type-1 Vectors Cleave SNARE Proteins and Inhibit CGRP Release in Rat Sensory Neurons.Toxins (Basel). 2019 Feb 19;11(2):123. doi: 10.3390/toxins11020123. Toxins (Basel). 2019. PMID: 30791373 Free PMC article.
-
HSV-1-derived recombinant and amplicon vectors for gene transfer and gene therapy.Curr Gene Ther. 2005 Oct;5(5):445-58. doi: 10.2174/156652305774329285. Curr Gene Ther. 2005. PMID: 16250886 Review.
Cited by
-
Promising Experimental Treatment in Animal Models and Human Studies of Interstitial Cystitis/Bladder Pain Syndrome.Int J Mol Sci. 2024 Jul 23;25(15):8015. doi: 10.3390/ijms25158015. Int J Mol Sci. 2024. PMID: 39125584 Free PMC article. Review.
-
Non-replicative herpes simplex virus genomic and amplicon vectors for gene therapy - an update.Gene Ther. 2025 May;32(3):173-183. doi: 10.1038/s41434-024-00500-x. Epub 2024 Nov 12. Gene Ther. 2025. PMID: 39533042 Free PMC article. Review.
-
Approaches and applications in transdermal and transpulmonary gene drug delivery.Front Bioeng Biotechnol. 2025 Jan 15;12:1519557. doi: 10.3389/fbioe.2024.1519557. eCollection 2024. Front Bioeng Biotechnol. 2025. PMID: 39881959 Free PMC article. Review.
References
-
- Ruffion A., Castro-Diaz D., Patel H., Khalaf K., Onyenwenyi A., Globe D., LeReun C., Teneishvili M., Edwards M. Systematic Review of the Epidemiology of Urinary Incontinence and Detrusor Overactivity among Patients with Neurogenic Overactive Bladder. Neuroepidemiology. 2013;41:146–155. doi: 10.1159/000353274. - DOI - PubMed
-
- Ginsberg D., Gousse A., Keppenne V., Sievert K.-D., Thompson C., Lam W., Brin M.F., Jenkins B., Haag-Molkenteller C. Phase 3 Efficacy and Tolerability Study of OnabotulinumtoxinA for Urinary Incontinence from Neurogenic Detrusor Overactivity. J. Urol. 2012;187:2131–2139. doi: 10.1016/j.juro.2012.01.125. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

