Involvement of Auxin-Mediated CqEXPA50 Contributes to Salt Tolerance in Quinoa (Chenopodium quinoa) by Interaction with Auxin Pathway Genes
- PMID: 35955612
- PMCID: PMC9369402
- DOI: 10.3390/ijms23158480
Involvement of Auxin-Mediated CqEXPA50 Contributes to Salt Tolerance in Quinoa (Chenopodium quinoa) by Interaction with Auxin Pathway Genes
Abstract
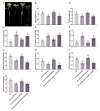
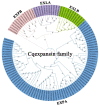
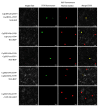
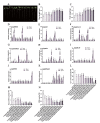
Soil salinization is a global problem that limits crop yields and threatens agricultural development. Auxin-induced expansins contribute to plant salt tolerance through cell wall loosening. However, how auxins and expansins contribute to the adaptation of the halophyte quinoa (Chenopodium quinoa) to salt stress has not yet been reported. Here, auxin was found to contribute to the salt tolerance of quinoa by promoting the accumulation of photosynthetic pigments under salt stress, maintaining enzymatic and nonenzymatic antioxidant systems and scavenging excess reactive oxygen species (ROS). The Chenopodium quinoa expansin (Cqexpansin) family and the auxin pathway gene family (Chenopodium quinoa auxin response factor (CqARF), Chenopodium quinoa auxin/indoleacetic acid (CqAux/IAA), Chenopodium quinoa Gretchen Hagen 3 (CqGH3) and Chenopodium quinoa small auxin upregulated RNA (CqSAUR)) were identified from the quinoa genome. Combined expression profiling identified Chenopodium quinoa α-expansin 50 (CqEXPA50) as being involved in auxin-mediated salt tolerance. CqEXPA50 enhanced salt tolerance in quinoa seedlings was revealed by transient overexpression and physiological and biochemical analyses. Furthermore, the auxin pathway and salt stress-related genes regulated by CqEXPA50 were identified. The interaction of CqEXPA50 with these proteins was demonstrated by bimolecular fluorescence complementation (BIFC). The proteins that interact with CqEXPA50 were also found to improve salt tolerance. In conclusion, this study identified some genes potentially involved in the salt tolerance regulatory network of quinoa, providing new insights into salt tolerance.
Keywords: Cqexpansin; antioxidant capacity; auxin; auxin pathway gene; salt stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
CqZF-HD14 enhances drought tolerance in quinoa seedlings through interaction with CqHIPP34 and CqNAC79.Plant Sci. 2022 Oct;323:111406. doi: 10.1016/j.plantsci.2022.111406. Epub 2022 Aug 3. Plant Sci. 2022. PMID: 35931235
-
Malate-mediated CqMADS68 enhances aluminum tolerance in quinoa seedlings through interaction with CqSTOP6, CqALMT6 and CqWRKY88.J Hazard Mater. 2022 Oct 5;439:129630. doi: 10.1016/j.jhazmat.2022.129630. Epub 2022 Jul 19. J Hazard Mater. 2022. PMID: 35872459
-
Transcriptome analysis and differential gene expression profiling of two contrasting quinoa genotypes in response to salt stress.BMC Plant Biol. 2020 Dec 30;20(1):568. doi: 10.1186/s12870-020-02753-1. BMC Plant Biol. 2020. PMID: 33380327 Free PMC article.
-
Expansins in Salt and Drought Stress Adaptation: From Genome-Wide Identification to Functional Characterisation in Crops.Plants (Basel). 2025 Apr 28;14(9):1327. doi: 10.3390/plants14091327. Plants (Basel). 2025. PMID: 40364355 Free PMC article. Review.
-
[Domesticated cultivation and genetic breeding of Chenopodium quinoa].Yi Chuan. 2019 Nov 20;41(11):1009-1022. doi: 10.16288/j.yczz.19-177. Yi Chuan. 2019. PMID: 31735704 Review. Chinese.
Cited by
-
Populus trichocarpa EXPA6 Facilitates Radial and Longitudinal Transport of Na+ under Salt Stress.Int J Mol Sci. 2024 Aug 29;25(17):9354. doi: 10.3390/ijms25179354. Int J Mol Sci. 2024. PMID: 39273303 Free PMC article.
-
A chromosome-scale assembly of the quinoa genome provides insights into the structure and dynamics of its subgenomes.Commun Biol. 2023 Dec 13;6(1):1263. doi: 10.1038/s42003-023-05613-4. Commun Biol. 2023. PMID: 38092895 Free PMC article.
-
Emerging applications of gene editing technologies for the development of climate-resilient crops.Front Genome Ed. 2025 Mar 10;7:1524767. doi: 10.3389/fgeed.2025.1524767. eCollection 2025. Front Genome Ed. 2025. PMID: 40129518 Free PMC article. Review.
-
Genome-Wide Identification, Phylogenetic and Expression Analysis of Expansin Gene Family in Medicago sativa L.Int J Mol Sci. 2024 Apr 25;25(9):4700. doi: 10.3390/ijms25094700. Int J Mol Sci. 2024. PMID: 38731920 Free PMC article.
-
Comprehensive Review on Bimolecular Fluorescence Complementation and Its Application in Deciphering Protein-Protein Interactions in Cell Signaling Pathways.Biomolecules. 2024 Jul 17;14(7):859. doi: 10.3390/biom14070859. Biomolecules. 2024. PMID: 39062573 Free PMC article. Review.
References
-
- Ruan C.J., Teixeira da Silva J.A., Mopper S., Qind P., Lutts S. Halophyte improvement for a salinized world. Crit. Rev. Plant Sci. 2010;29:329–359. doi: 10.1080/07352689.2010.524517. - DOI
-
- Zelm E.V., Zhang Y.X., Testerink C. Salt tolerance mechanisms of plants. Annu. Rev. Plant. Biol. 2020;71:1–31. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

