Comparative Transcriptomic Analysis of Root and Leaf Transcript Profiles Reveals the Coordinated Mechanisms in Response to Salinity Stress in Common Vetch
- PMID: 35955619
- PMCID: PMC9369433
- DOI: 10.3390/ijms23158477
Comparative Transcriptomic Analysis of Root and Leaf Transcript Profiles Reveals the Coordinated Mechanisms in Response to Salinity Stress in Common Vetch
Abstract
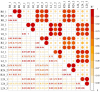
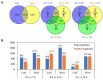
Owing to its strong environmental suitability to adverse abiotic stress conditions, common vetch (Vicia sativa) is grown worldwide for both forage and green manure purposes and is an important protein source for human consumption and livestock feed. The germination of common vetch seeds and growth of seedlings are severely affected by salinity stress, and the response of common vetch to salinity stress at the molecular level is still poorly understood. In this study, we report the first comparative transcriptomic analysis of the leaves and roots of common vetch under salinity stress. A total of 6361 differentially expressed genes were identified in leaves and roots. In the roots, the stress response was dominated by genes involved in peroxidase activity. However, the genes in leaves focused mainly on Ca2+ transport. Overexpression of six salinity-inducible transcription factors in yeast further confirmed their biological functions in the salinity stress response. Our study provides the most comprehensive transcriptomic analysis of common vetch leaf and root responses to salinity stress. Our findings broaden the knowledge of the common and distinct intrinsic molecular mechanisms within the leaves and roots of common vetch and could help to develop common vetch cultivars with high salinity tolerance.
Keywords: common vetch; full-length transcripts; leaf; root; salinity stress; yeast.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures










Similar articles
-
Designing Novel Strategies for Improving Old Legumes: An Overview from Common Vetch.Plants (Basel). 2023 Mar 10;12(6):1275. doi: 10.3390/plants12061275. Plants (Basel). 2023. PMID: 36986962 Free PMC article. Review.
-
Coordinated mechanisms of leaves and roots in response to drought stress underlying full-length transcriptome profiling in Vicia sativa L.BMC Plant Biol. 2020 Apr 15;20(1):165. doi: 10.1186/s12870-020-02358-8. BMC Plant Biol. 2020. PMID: 32293274 Free PMC article.
-
Impact of priming on seed germination, seedling growth and gene expression in common vetch under salinity stress.Cell Mol Biol (Noisy-le-grand). 2019 Mar 29;65(3):18-24. Cell Mol Biol (Noisy-le-grand). 2019. PMID: 30942152
-
Evaluation of salt tolerance in common vetch (Vicia sativa L.) germplasms and the physiological responses to salt stress.J Plant Physiol. 2022 Nov;278:153811. doi: 10.1016/j.jplph.2022.153811. Epub 2022 Sep 12. J Plant Physiol. 2022. PMID: 36126616
-
A comparative transcriptomic analysis reveals a coordinated mechanism activated in response to cold acclimation in common vetch (Vicia sativa L.).BMC Genomics. 2022 Dec 8;23(1):814. doi: 10.1186/s12864-022-09039-w. BMC Genomics. 2022. PMID: 36482290 Free PMC article.
Cited by
-
Screening and identification of multiple abiotic stress responsive candidate genes based on hybrid-sequencing in Vicia sativa.Heliyon. 2023 Feb 4;9(2):e13536. doi: 10.1016/j.heliyon.2023.e13536. eCollection 2023 Feb. Heliyon. 2023. PMID: 36816321 Free PMC article.
-
Designing Novel Strategies for Improving Old Legumes: An Overview from Common Vetch.Plants (Basel). 2023 Mar 10;12(6):1275. doi: 10.3390/plants12061275. Plants (Basel). 2023. PMID: 36986962 Free PMC article. Review.
-
Transcriptome Profiling Reveals Molecular Responses to Salt Stress in Common Vetch (Vicia sativa L.).Plants (Basel). 2024 Mar 3;13(5):714. doi: 10.3390/plants13050714. Plants (Basel). 2024. PMID: 38475559 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

