Bile Acid-Drug Interaction via Organic Anion-Transporting Polypeptide 4C1 Is a Potential Mechanism of Altered Pharmacokinetics of Renally Excreted Drugs
- PMID: 35955643
- PMCID: PMC9369231
- DOI: 10.3390/ijms23158508
Bile Acid-Drug Interaction via Organic Anion-Transporting Polypeptide 4C1 Is a Potential Mechanism of Altered Pharmacokinetics of Renally Excreted Drugs
Abstract
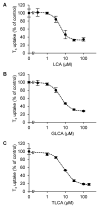
Patients with liver diseases not only experience the adverse effects of liver-metabolized drugs, but also the unexpected adverse effects of renally excreted drugs. Bile acids alter the expression of renal drug transporters, however, the direct effects of bile acids on drug transport remain unknown. Renal drug transporter organic anion-transporting polypeptide 4C1 (OATP4C1) was reported to be inhibited by chenodeoxycholic acid. Therefore, we predicted that the inhibition of OATP4C1-mediated transport by bile acids might be a potential mechanism for the altered pharmacokinetics of renally excreted drugs. We screened 45 types of bile acids and calculated the IC50, Ki values, and bile acid−drug interaction (BDI) indices of bile acids whose inhibitory effect on OATP4C1 was >50%. From the screening results, lithocholic acid (LCA), glycine-conjugated lithocholic acid (GLCA), and taurine-conjugated lithocholic acid (TLCA) were newly identified as inhibitors of OATP4C1. Since the BDI index of LCA was 0.278, LCA is likely to inhibit OATP4C1-mediated transport in clinical settings. Our findings suggest that dose adjustment of renally excreted drugs may be required in patients with renal failure as well as in patients with hepatic failure. We believe that our findings provide essential information for drug development and safe drug treatment in clinics.
Keywords: OATP4C1; bile acids; bile acid–drug interaction; lithocholic acid; liver disease; renally excreted drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Narisue M., Sugimoto Y., Shibata R., Otsubo T., Hirata S. High Incidence of Adverse Events in Patients with Impaired Renal Function Taking Pregabalin, Even When Dose Is Adjusted for Renal Function. Nihon Toseki Igakkai Zasshi. 2015;48:155–161. doi: 10.4009/jsdt.48.155. - DOI
-
- Fujita K.I., Masuo Y., Okumura H., Watanabe Y., Suzuki H., Sunakawa Y., Shimada K., Kawara K., Akiyama Y., Kitamura M., et al. Increased Plasma Concentrations of Unbound SN-38, the Active Metabolite of Irinotecan, in Cancer Patients with Severe Renal Failure. Pharm. Res. 2016;33:269–282. doi: 10.1007/s11095-015-1785-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

