The Significance of Cell Surface N-Glycosylation for Internalization and Potency of Cytotoxic Conjugates Targeting Receptor Tyrosine Kinases
- PMID: 35955648
- PMCID: PMC9368766
- DOI: 10.3390/ijms23158514
The Significance of Cell Surface N-Glycosylation for Internalization and Potency of Cytotoxic Conjugates Targeting Receptor Tyrosine Kinases
Abstract
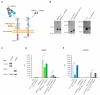
Precise anticancer therapies employing cytotoxic conjugates constitute a side-effect-limited, highly attractive alternative to commonly used cancer treatment modalities, such as conventional chemotherapy, radiotherapy or surgical interventions. Receptor tyrosine kinases are a large family of N-glycoproteins intensively studied as molecular targets for cytotoxic conjugates in various cancers. At the cell surface, these receptors are embedded in a dense carbohydrate layer formed by numerous plasma membrane glycoproteins. The complexity of the cell surface architecture is further increased by galectins, secreted lectins capable of recognizing and clustering glycoconjugates, affecting their motility and activity. Cell surface N-glycosylation is intensively remodeled by cancer cells; however, the contribution of this phenomenon to the efficiency of treatment with cytotoxic conjugates is largely unknown. Here, we evaluated the significance of N-glycosylation for the internalization and toxicity of conjugates targeting two model receptor tyrosine kinases strongly implicated in cancer: HER2 and FGFR1. We employed three conjugates of distinct molecular architecture and specificity: AffibodyHER2-vcMMAE (targeting HER2), vcMMAE-KCK-FGF1.E and T-Fc-vcMMAE (recognizing different epitopes within FGFR1). We demonstrated that inhibition of N-glycosylation reduced the cellular uptake of all conjugates tested and provided evidence for a role of the galectin network in conjugate internalization. In vitro binding studies revealed that the reduced uptake of conjugates is not due to impaired HER2 and FGFR1 binding. Importantly, we demonstrated that alteration of N-glycosylation can affect the cytotoxic potential of conjugates. Our data implicate a key role for cell surface N-glycosylation in the delivery of cytotoxic conjugates into cancer cells.
Keywords: N-glycosylation; RTK; cancer therapy; cytotoxic conjugates; endocytosis; galectins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
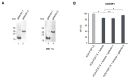


Similar articles
-
The cytotoxic conjugate of highly internalizing tetravalent antibody for targeting FGFR1-overproducing cancer cells.Mol Med. 2021 May 7;27(1):46. doi: 10.1186/s10020-021-00306-2. Mol Med. 2021. PMID: 33962559 Free PMC article.
-
Modular self-assembly system for development of oligomeric, highly internalizing and potent cytotoxic conjugates targeting fibroblast growth factor receptors.J Biomed Sci. 2021 Oct 11;28(1):69. doi: 10.1186/s12929-021-00767-x. J Biomed Sci. 2021. PMID: 34635096 Free PMC article.
-
Targeting HER2 and FGFR-positive cancer cells with a bispecific cytotoxic conjugate combining anti-HER2 Affibody and FGF2.Int J Biol Macromol. 2024 Jan;254(Pt 1):127657. doi: 10.1016/j.ijbiomac.2023.127657. Epub 2023 Oct 28. Int J Biol Macromol. 2024. PMID: 38287563
-
Galectins as modulators of receptor tyrosine kinases signaling in health and disease.Cytokine Growth Factor Rev. 2021 Aug;60:89-106. doi: 10.1016/j.cytogfr.2021.03.004. Epub 2021 Mar 27. Cytokine Growth Factor Rev. 2021. PMID: 33863623 Review.
-
Targeted cancer therapy: conferring specificity to cytotoxic drugs.Acc Chem Res. 2008 Jan;41(1):98-107. doi: 10.1021/ar700108g. Epub 2007 Aug 18. Acc Chem Res. 2008. PMID: 17705444 Review.
Cited by
-
N-glycosylation acts as a switch for FGFR1 trafficking between the plasma membrane and nuclear envelope.Cell Commun Signal. 2023 Jul 21;21(1):177. doi: 10.1186/s12964-023-01203-3. Cell Commun Signal. 2023. PMID: 37480072 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

