The Role of Histology-Agnostic Drugs in the Treatment of Metastatic Castration-Resistant Prostate Cancer
- PMID: 35955671
- PMCID: PMC9369092
- DOI: 10.3390/ijms23158535
The Role of Histology-Agnostic Drugs in the Treatment of Metastatic Castration-Resistant Prostate Cancer
Abstract
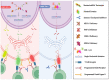
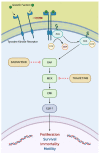
Precision medicine has opened up a new era in the development of anti-cancer agents that is focused on identifying biomarkers predictive of treatment response regardless of tumor histology. Since 2017, the Food and Drug Administration has approved six drugs with histology-agnostic indications: pembrolizumab (both for tumors with the mismatch-repair deficiency (dMMR)/high microsatellite instability (MSI-H) phenotype and for those with the high tumor mutational burden (TMB-H) phenotype), dostarlimab (for dMMR tumors), larotrectinib and entrectinib (for tumors harboring neurotrophic tyrosine receptor kinase (NTRK) fusions), and the combination of dabrafenib plus trametinib (for BRAF V600E-mutated tumors). The genomic alterations targeted by these antineoplastic agents are rare in metastatic castration-resistant prostate cancer (mCRPC). Furthermore, only a small number of mCRPC patients were enrolled in the clinical trials that led to the approval of the above-mentioned drugs. Therefore, we critically reviewed the literature on the efficacy of histology-agnostic drugs in mCRPC patients. Although the available evidence derives from retrospective studies and case reports, our results confirmed the efficacy of pembrolizumab in dMMR/MSI-H mCRPC. In contrast, few data are available for dostarlimab, larotrectinib, entrectinib, and dabrafenib-trametinib in this subset of patients. Large, multi-institutional registries aimed at collecting real-world data are needed to better comprehend the role of tissue-agnostic drugs in mCRPC patients.
Keywords: BRAF V600E; MSI-H; NTRK; TMB-H; dMMR; histology-agnostic; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- De Bono J.S., Oudard S., Ozguroglu M., Hansen S., Machiels J.-P., Kocak I., Gravis G., Bodrogi I., Mackenzie M.J., Shen L., et al. Prednisone plus Cabazitaxel or Mitoxantrone for Metastatic Castration-Resistant Prostate Cancer Progressing after Docetaxel Treatment: A Randomised Open-Label Trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

