Utilization of Aminoguanidine Prevents Cytotoxic Effects of Semen
- PMID: 35955696
- PMCID: PMC9369337
- DOI: 10.3390/ijms23158563
Utilization of Aminoguanidine Prevents Cytotoxic Effects of Semen
Abstract
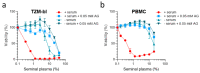
Studies of human semen in cell or tissue culture are hampered by the high cytotoxic activity of this body fluid. The components responsible for the cell damaging activity of semen are amine oxidases, which convert abundant polyamines, such as spermine or spermidine in seminal plasma into toxic intermediates. Amine oxidases are naturally present at low concentrations in seminal plasma and at high concentrations in fetal calf serum, a commonly used cell culture supplement. Here, we show that, in the presence of fetal calf serum, seminal plasma, as well as the polyamines spermine and spermidine, are highly cytotoxic to immortalized cells, primary blood mononuclear cells, and vaginal tissue. Thus, experiments investigating the effect of polyamines and seminal plasma on cellular functions should be performed with great caution, considering the confounding cytotoxic effects. The addition of the amine oxidase inhibitor aminoguanidine to fetal calf serum and/or the utilization of serum-free medium greatly reduced this serum-induced cytotoxicity of polyamines and seminal plasma in cell lines, primary cells, and tissues and, thus, should be implemented in all future studies analyzing the role of polyamines and semen on cellular functions.
Keywords: cytotoxicity; polyamines; seminal fluid; spermine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Valsa J., Skandhan K.P., Sumangala B., Jaya V. Time Bound Changes (in 24 h) in Human Sperm Motility and Level of Calcium and Magnesium in Seminal Plasma. Alex. J. Med. 2016;52:235–241. doi: 10.1016/j.ajme.2015.09.005. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

