Cellular Redox Metabolism Is Modulated by the Distinct Localization of Cyclic Nucleotide Phosphodiesterase 5A Isoforms
- PMID: 35955722
- PMCID: PMC9368758
- DOI: 10.3390/ijms23158587
Cellular Redox Metabolism Is Modulated by the Distinct Localization of Cyclic Nucleotide Phosphodiesterase 5A Isoforms
Abstract
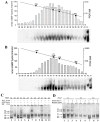
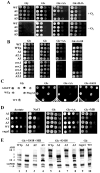
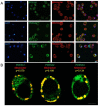
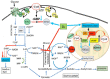
3'-5' cyclic nucleotide phosphodiesterases (PDEs) are a family of evolutionarily conserved cAMP and/or cGMP hydrolyzing enzymes, components of transduction pathways regulating crucial aspects of cell life. Among them, cGMP-specific PDE5-being a regulator of vascular smooth muscle contraction-is the molecular target of several drugs used to treat erectile dysfunction and pulmonary hypertension. Production of full-length murine PDE5A isoforms in the milk-yeast Kluyveromyces lactis showed that the quaternary assembly of MmPDE5A1 is a mixture of dimers and tetramers, while MmPDE5A2 and MmPDE5A3 only assembled as dimers. We showed that the N-terminal peptide is responsible for the tetramer assembly of MmPDE5A1, while that of the MmPDE5A2 is responsible for its mitochondrial localization. Overexpression of the three isoforms alters at different levels the cAMP/cGMP equilibrium as well as the NAD(P)+/NAD(P)H balance and induces a metabolic switch from oxidative to fermentative. In particular, the mitochondrial localization of MmPDE5A2 unveiled the existence of a cAMP-cGMP signaling cascade in this organelle, for which we propose a metabolic model that could explain the role of PDE5 in some cardiomyopathies and some of the side effects of its inhibitors.
Keywords: Kluyveromyces lactis; cGMP-specific phosphodiesterase; glycolytic/respiratory flux; rag phenotype; redox balance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Beavo J.A., Conti M., Heaslip R.J. Multiple cyclic nucleotide phosphodiesterases. Mol. Pharmacol. 1994;46:399–405. - PubMed
-
- Keravis T., Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic development. Br. J. Pharmacol. 2012;165:1288–1305. doi: 10.1111/j.1476-5381.2011.01729.x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

