VIP/VPAC Axis Expression in Immune-Mediated Inflammatory Disorders: Associated miRNA Signatures
- PMID: 35955723
- PMCID: PMC9369218
- DOI: 10.3390/ijms23158578
VIP/VPAC Axis Expression in Immune-Mediated Inflammatory Disorders: Associated miRNA Signatures
Abstract
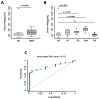
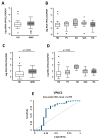
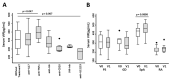
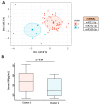
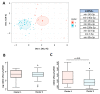
Few studies have considered immune-mediated inflammatory disorders (IMID) together, which is necessary to adequately understand them given they share common mechanisms. Our goal was to investigate the expression of vasoactive intestinal peptide (VIP) and its receptors VPAC1 and VPAC2 in selected IMID, analyze the effect of biological therapies on them, and identify miRNA signatures associated with their expression. Serum VIP levels and mRNA of VPAC and miRNA expression in peripheral blood mononuclear cells were analyzed from 52 patients with psoriasis, rheumatoid arthritis, Graves’ disease, or spondyloarthritis and from 38 healthy subjects. IMID patients showed higher levels of VIP and increased expression of VPAC2 compared to controls (p < 0.0001 and p < 0.0192, respectively). Receiver operating characteristic curve analysis showed that the levels of VIP or VPAC2 expression were adequate discriminators capable of identifying IMID. Treatment of IMID patients with anti-TNFα and anti-IL12/23 significantly affected serum VIP levels. We identified miRNA signatures associated with levels of serum VIP and VPAC2 expression, which correlated with IMID diagnosis of the patients. The results indicate that the expression of VIP/VPAC2 is able of identify IMIDs and open up a line of research based on the association between the VIP/VPAC axis and miRNA signatures in immune-mediated diseases.
Keywords: Graves’ disease; VIP; VPAC receptors; immune-mediated inflammatory disorders; microRNAs; psoriasis; rheumatoid arthritis; spondyloarthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

