Beyond Cancer: Regulation and Function of PD-L1 in Health and Immune-Related Diseases
- PMID: 35955729
- PMCID: PMC9369208
- DOI: 10.3390/ijms23158599
Beyond Cancer: Regulation and Function of PD-L1 in Health and Immune-Related Diseases
Abstract
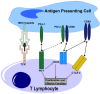
Programmed Cell Death 1 Ligand 1 (PD-L1, CD274, B7-H1) is a transmembrane protein which is strongly involved in immune modulation, serving as checkpoint regulator. Interaction with its receptor, Programmed Cell Death Protein 1 (PD-1), induces an immune-suppressive signal, which modulates the activity of T cells and other effector cells. This mediates peripheral tolerance and contributes to tumor immune escape. PD-L1 became famous due to its deployment in cancer therapy, where blockage of PD-L1 with the help of therapeutic antagonistic antibodies achieved impressive clinical responses by reactivating effector cell functions against tumor cells. Therefore, in the past, the focus has been placed on PD-L1 expression and its function in various malignant cells, whereas its role in healthy tissue and diseases apart from cancer remained largely neglected. In this review, we summarize the function of PD-L1 in non-cancerous cells, outlining its discovery and origin, as well as its involvement in different cellular and immune-related processes. We provide an overview of transcriptional and translational regulation, and expression patterns of PD-L1 in different cells and organs, and illuminate the involvement of PD-L1 in different autoimmune diseases as well as in the context of transplantation and pregnancy.
Keywords: PD-1–PD-L1 axis; PD-L1; immune checkpoint; non-cancerous tissues.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Peterson E.J., Maltzman J.S. 12—T-Cell Activation and Tolerance. In: Rich R.R., Fleisher T.A., Shearer W.T., Schroeder H.W., Frew A.J., Weyand C.M., editors. Clinical Immunology. 5th ed. Elsevier; London, UK: 2019. pp. 183–196.e181. - DOI
-
- Wei S.C., Duffy C.R., Allison J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069–1086. doi: 10.1158/2159-8290.CD-18-0367. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

