Downregulation of IL-8 and IL-10 by the Activation of Ca2+-Activated K+ Channel KCa3.1 in THP-1-Derived M2 Macrophages
- PMID: 35955737
- PMCID: PMC9368915
- DOI: 10.3390/ijms23158603
Downregulation of IL-8 and IL-10 by the Activation of Ca2+-Activated K+ Channel KCa3.1 in THP-1-Derived M2 Macrophages
Abstract
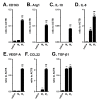
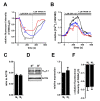
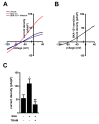
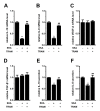
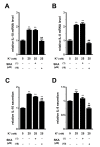
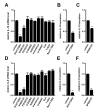
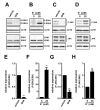
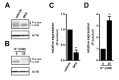
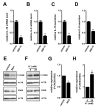
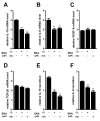
THP-1-differentiated macrophages are useful for investigating the physiological significance of tumor-associated macrophages (TAMs). In the tumor microenvironment (TME), TAMs with the M2-like phenotype play a critical role in promoting cancer progression and metastasis by inhibiting the immune surveillance system. We examined the involvement of Ca2+-activated K+ channel KCa3.1 in TAMs in expressing pro-tumorigenic cytokines and angiogenic growth factors. In THP-1-derived M2 macrophages, the expression levels of IL-8 and IL-10 were significantly decreased by treatment with the selective KCa3.1 activator, SKA-121, without changes in those of VEGF and TGF-β1. Furthermore, under in vitro experimental conditions that mimic extracellular K+ levels in the TME, IL-8 and IL-10 levels were both significantly elevated, and these increases were reversed by combined treatment with SKA-121. Among several signaling pathways potentially involved in the transcriptional regulation of IL-8 and IL-10, respective treatments with ERK and JNK inhibitors significantly repressed their transcriptions, and treatment with SKA-121 significantly reduced the phosphorylated ERK, JNK, c-Jun, and CREB levels. These results strongly suggest that the KCa3.1 activator may suppress IL-10-induced tumor immune surveillance escape and IL-8-induced tumorigenicity and metastasis by inhibiting their production from TAMs through ERK-CREB and JNK-c-Jun cascades.
Keywords: IL-10; IL-8; K+ channel; KCa3.1; THP-1; tumor microenvironment; tumor-associated macrophage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

