Synthesis and Immunological Evaluation of Mannosylated Desmuramyl Dipeptides Modified by Lipophilic Triazole Substituents
- PMID: 35955759
- PMCID: PMC9368957
- DOI: 10.3390/ijms23158628
Synthesis and Immunological Evaluation of Mannosylated Desmuramyl Dipeptides Modified by Lipophilic Triazole Substituents
Abstract
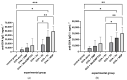
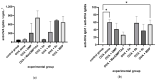
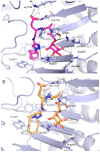
Muramyl dipeptide (N-acetylmuramyl-L-alanyl-D-isoglutamine, MDP) is the smallest peptidoglycan fragment able to trigger an immune response by activating the NOD2 receptor. Structural modification of MDP can lead to analogues with improved immunostimulating properties. The aim of this work was to prepare mannosylated desmuramyl peptides (ManDMP) containing lipophilic triazole substituents to study their immunomodulating activities in vivo. The adjuvant activity of the prepared compounds was evaluated in the mouse model using ovalbumin as an antigen and compared to the MDP and referent adjuvant ManDMPTAd. The obtained results confirm that the α-position of D-isoGln is the best position for the attachment of lipophilic substituents, especially adamantylethyl triazole. Compound 6c exhibited the strongest adjuvant activity, comparable to the MDP and better than referent ManDMPTAd.
Keywords: desmuramyl peptide; immunostimulating activity; mannose; triazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Design, Synthesis, and Biological Evaluation of Desmuramyl Dipeptides Modified by Adamantyl-1,2,3-triazole.Molecules. 2021 Oct 21;26(21):6352. doi: 10.3390/molecules26216352. Molecules. 2021. PMID: 34770761 Free PMC article.
-
Design, synthesis and biological evaluation of immunostimulating mannosylated desmuramyl peptides.Beilstein J Org Chem. 2019 Jul 29;15:1805-1814. doi: 10.3762/bjoc.15.174. eCollection 2019. Beilstein J Org Chem. 2019. PMID: 31467600 Free PMC article.
-
Di-mannosylation enhances the adjuvant properties of adamantane-containing desmuramyl peptides in vivo.Org Biomol Chem. 2024 Aug 14;22(32):6506-6519. doi: 10.1039/d4ob00592a. Org Biomol Chem. 2024. PMID: 38884368
-
Activation of immune responses by muroctasin.Arzneimittelforschung. 1988 Jul;38(7A):976-7. Arzneimittelforschung. 1988. PMID: 3056427 Review.
-
[The immunostimulating activity of muramyl dipeptide and its derivatives].Zh Mikrobiol Epidemiol Immunobiol. 1999 May-Jun;(3):104-10. Zh Mikrobiol Epidemiol Immunobiol. 1999. PMID: 10852007 Review. Russian. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

