Intrathecal Actions of the Cannabis Constituents Δ(9)-Tetrahydrocannabinol and Cannabidiol in a Mouse Neuropathic Pain Model
- PMID: 35955774
- PMCID: PMC9369424
- DOI: 10.3390/ijms23158649
Intrathecal Actions of the Cannabis Constituents Δ(9)-Tetrahydrocannabinol and Cannabidiol in a Mouse Neuropathic Pain Model
Abstract
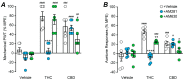
(1) Background: The psychoactive and non-psychoactive constituents of cannabis, Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), synergistically reduce allodynia in various animal models of neuropathic pain. Unfortunately, THC-containing drugs also produce substantial side-effects when administered systemically. We examined the effectiveness of targeted spinal delivery of these cannabis constituents, alone and in combination. (2) Methods: The effect of acute intrathecal drug delivery on allodynia and common cannabinoid-like side-effects was examined in a mouse chronic constriction injury (CCI) model of neuropathic pain. (3) Results: intrathecal THC and CBD produced dose-dependent reductions in mechanical and cold allodynia. In a 1:1 combination, they synergistically reduced mechanical and cold allodynia, with a two-fold increase in potency compared to their predicted additive effect. Neither THC, CBD nor combination THC:CBD produced any cannabis-like side-effects at equivalent doses. The anti-allodynic effects of THC were abolished and partly reduced by cannabinoid CB1 and CB2 receptor antagonists AM281 and AM630, respectively. The anti-allodynic effects of CBD were partly reduced by AM630. (4) Conclusions: these findings indicate that intrathecal THC and CBD, individually and in combination, could provide a safe and effective treatment for nerve injury induced neuropathic pain.
Keywords: THC; cannabidiol; cannabinoid; intrathecal; mice; neuropathic pain; synergy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




Similar articles
-
Cannabis constituent synergy in a mouse neuropathic pain model.Pain. 2017 Dec;158(12):2452-2460. doi: 10.1097/j.pain.0000000000001051. Pain. 2017. PMID: 28885457
-
Oral efficacy of Δ(9)-tetrahydrocannabinol and cannabidiol in a mouse neuropathic pain model.Neuropharmacology. 2021 May 15;189:108529. doi: 10.1016/j.neuropharm.2021.108529. Epub 2021 Mar 16. Neuropharmacology. 2021. PMID: 33741405
-
THC and gabapentin interactions in a mouse neuropathic pain model.Neuropharmacology. 2019 Jan;144:115-121. doi: 10.1016/j.neuropharm.2018.10.006. Epub 2018 Oct 9. Neuropharmacology. 2019. PMID: 30312630
-
Antinociceptive effects of minor cannabinoids, terpenes and flavonoids in Cannabis.Behav Pharmacol. 2022 Apr 1;33(2&3):130-157. doi: 10.1097/FBP.0000000000000627. Behav Pharmacol. 2022. PMID: 33709984 Review.
-
Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Δ9-Tetrahydrocannabinol.Neuropsychopharmacology. 2018 Jan;43(1):142-154. doi: 10.1038/npp.2017.209. Epub 2017 Sep 6. Neuropsychopharmacology. 2018. PMID: 28875990 Free PMC article. Review.
Cited by
-
Beyond CBD: Inhibitory effects of lesser studied phytocannabinoids on human voltage-gated sodium channels.Front Physiol. 2023 Feb 20;14:1081186. doi: 10.3389/fphys.2023.1081186. eCollection 2023. Front Physiol. 2023. PMID: 36891145 Free PMC article.
-
Analgesia by intrathecal delta-9-tetrahydrocannabinol is dependent on Cav3.2 calcium channels.Mol Brain. 2023 May 25;16(1):47. doi: 10.1186/s13041-023-01036-8. Mol Brain. 2023. PMID: 37231418 Free PMC article.
-
High-CBD Cannabis Vapor Attenuates Opioid Reward and Partially Modulates Nociception in Female Rats.Addict Neurosci. 2023 Mar;5:100050. doi: 10.1016/j.addicn.2022.100050. Epub 2022 Dec 5. Addict Neurosci. 2023. PMID: 36937502 Free PMC article.
-
Antihyperalgesic effect of joint mobilization requires Cav3.2 calcium channels.Mol Brain. 2023 Jul 18;16(1):60. doi: 10.1186/s13041-023-01049-3. Mol Brain. 2023. PMID: 37464359 Free PMC article.
-
A type II cannabis extract and a 1:1 blend of Δ(9)-tetrahydrocannabinol and cannabidiol display distinct antinociceptive profiles and engage different endocannabinoid targets when administered into the subarachnoid space.Front Pharmacol. 2023 Sep 8;14:1235255. doi: 10.3389/fphar.2023.1235255. eCollection 2023. Front Pharmacol. 2023. PMID: 37745077 Free PMC article.
References
-
- Dworkin R.H., O’Connor A.B., Audette J., Baron R., Gourlay G.K., Haanpaa M.L., Kent J.L., Krane E.J., Lebel A.A., Levy R.M., et al. Recommendations for the pharmacological management of neuropathic pain: An overview and literature update. Mayo Clin. Proc. 2010;85:S3–S14. doi: 10.4065/mcp.2009.0649. - DOI - PMC - PubMed
-
- Stockings E., Campbell G., Hall W.D., Nielsen S., Zagic D., Rahman R., Murnion B., Farrell M., Weier M., Degenhardt L. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: A systematic review and meta-analysis of controlled and observational studies. Pain. 2018;159:1932–1954. doi: 10.1097/j.pain.0000000000001293. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

