Harnessing the Power of Purple Sweet Potato Color and Myo-Inositol to Treat Classic Galactosemia
- PMID: 35955788
- PMCID: PMC9369367
- DOI: 10.3390/ijms23158654
Harnessing the Power of Purple Sweet Potato Color and Myo-Inositol to Treat Classic Galactosemia
Abstract
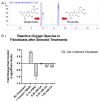
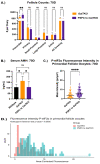
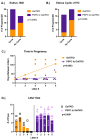
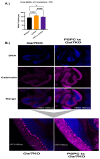
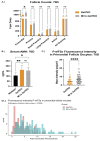
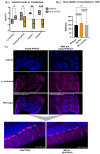
Classic Galactosemia (CG) is a devastating inborn error of the metabolism caused by mutations in the GALT gene encoding the enzyme galactose-1 phosphate uridylyltransferase in galactose metabolism. Severe complications of CG include neurological impairments, growth restriction, cognitive delays, and, for most females, primary ovarian insufficiency. The absence of the GALT enzyme leads to an accumulation of aberrant galactose metabolites, which are assumed to be responsible for the sequelae. There is no treatment besides the restriction of dietary galactose, which does not halt the development of the complications; thus, additional treatments are sorely needed. Supplements have been used in other inborn errors of metabolism but are not part of the therapeutic regimen for CG. The goal of this study was to test two generally recognized as safe supplements (purple sweet potato color (PSPC) and myo-inositol (MI)) that may impact cellular pathways contributing to the complications in CG. Our group uses a GalT gene-trapped mouse model to study the pathophysiology in CG, which phenocopy many of the complications. Here we report the ability of PSPC to ameliorate dysregulation in the ovary, brain, and liver of our mutant mice as well as positive results of MI supplementation in the ovary and brain.
Keywords: Classic Galactosemia; Integrated Stress Response; antioxidant; cerebellum; eukaryotic initiation Factor 2 alpha (eIF2ɑ); hepatocyte balloon-cell change; myo-inositol; primary ovarian insufficiency; purple sweet potato color; supplements.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Assessment of Long-Term Safety and Efficacy of Purple Sweet Potato Color (PSPC) and Myo-Inositol (MI) Treatment for Motor Related and Behavioral Phenotypes in a Mouse Model of Classic Galactosemia.J Inherit Metab Dis. 2025 Mar;48(2):e70002. doi: 10.1002/jimd.70002. J Inherit Metab Dis. 2025. PMID: 39894675 Free PMC article.
-
Early postnatal alterations in follicular stress response and survival in a mouse model of Classic Galactosemia.J Ovarian Res. 2022 Nov 21;15(1):122. doi: 10.1186/s13048-022-01049-2. J Ovarian Res. 2022. PMID: 36414970 Free PMC article.
-
Is prenatal myo-inositol deficiency a mechanism of CNS injury in galactosemia?J Inherit Metab Dis. 2011 Apr;34(2):345-55. doi: 10.1007/s10545-010-9260-x. Epub 2011 Jan 19. J Inherit Metab Dis. 2011. PMID: 21246399 Review.
-
Novel mRNA-Based Therapy Reduces Toxic Galactose Metabolites and Overcomes Galactose Sensitivity in a Mouse Model of Classic Galactosemia.Mol Ther. 2020 Jan 8;28(1):304-312. doi: 10.1016/j.ymthe.2019.09.018. Epub 2019 Sep 19. Mol Ther. 2020. PMID: 31604675 Free PMC article.
-
Hereditary galactosemia.Metabolism. 2018 Jun;83:188-196. doi: 10.1016/j.metabol.2018.01.025. Epub 2018 Jan 31. Metabolism. 2018. PMID: 29409891 Review.
Cited by
-
Brain function in classic galactosemia, a galactosemia network (GalNet) members review.Front Genet. 2024 Feb 15;15:1355962. doi: 10.3389/fgene.2024.1355962. eCollection 2024. Front Genet. 2024. PMID: 38425716 Free PMC article. Review.
-
The hypergonadotropic hypogonadism conundrum of classic galactosemia.Hum Reprod Update. 2023 Mar 1;29(2):246-258. doi: 10.1093/humupd/dmac041. Hum Reprod Update. 2023. PMID: 36512573 Free PMC article.
-
Assessment of Long-Term Safety and Efficacy of Purple Sweet Potato Color (PSPC) and Myo-Inositol (MI) Treatment for Motor Related and Behavioral Phenotypes in a Mouse Model of Classic Galactosemia.J Inherit Metab Dis. 2025 Mar;48(2):e70002. doi: 10.1002/jimd.70002. J Inherit Metab Dis. 2025. PMID: 39894675 Free PMC article.
-
Primary ovarian insufficiency in Classic Galactosemia: a systematic review.J Endocrinol Invest. 2025 Jun;48(6):1303-1315. doi: 10.1007/s40618-024-02527-8. Epub 2025 Jan 16. J Endocrinol Invest. 2025. PMID: 39821528
-
Inositol in Disease and Development: Roles of Catabolism via myo-Inositol Oxygenase in Drosophila melanogaster.Int J Mol Sci. 2023 Feb 20;24(4):4185. doi: 10.3390/ijms24044185. Int J Mol Sci. 2023. PMID: 36835596 Free PMC article.
References
-
- Berry G.T. Classic Galactosemia and Clinical Variant Galactosemia. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., editors. GeneReviews(®) University of Washington; Seattle, WA, USA: 1993. - PubMed
-
- Kuiper A., Grünewald S., Murphy E., Coenen M.A., Eggink H., Zutt R., Rubio-Gozalbo M.E., Bosch A.M., Williams M., Derks T.G.J., et al. Movement disorders and nonmotor neuropsychological symptoms in children and adults with classical galactosemia. J. Inherit. Metab. Dis. 2019;42:451–458. doi: 10.1002/jimd.12054. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

