Sex-Dependent Regulation of Placental Oleic Acid and Palmitic Acid Metabolism by Maternal Glycemia and Associations with Birthweight
- PMID: 35955818
- PMCID: PMC9369035
- DOI: 10.3390/ijms23158685
Sex-Dependent Regulation of Placental Oleic Acid and Palmitic Acid Metabolism by Maternal Glycemia and Associations with Birthweight
Abstract
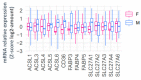
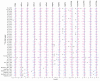
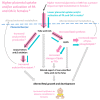
Pregnancy complications such as maternal hyperglycemia increase perinatal mortality and morbidity, but risks are higher in males than in females. We hypothesized that fetal sex-dependent differences in placental palmitic-acid (PA) and oleic-acid (OA) metabolism influence such risks. Placental explants (n = 22) were incubated with isotope-labeled fatty acids (13C-PA or 13C-OA) for 24 or 48 h and the production of forty-seven 13C-PA lipids and thirty-seven 13C-OA lipids quantified by LCMS. Linear regression was used to investigate associations between maternal glycemia, BMI and fetal sex with 13C lipids, and between 13C lipids and birthweight centile. Placental explants from females showed greater incorporation of 13C-OA and 13C-PA into almost all lipids compared to males. Fetal sex also influenced relationships with maternal glycemia, with many 13C-OA and 13C-PA acylcarnitines, 13C-PA-diacylglycerols and 13C-PA phospholipids positively associated with glycemia in females but not in males. In contrast, several 13C-OA triacylglycerols and 13C-OA phospholipids were negatively associated with glycemia in males but not in females. Birthweight centile in females was positively associated with six 13C-PA and three 13C-OA lipids (mainly acylcarnitines) and was negatively associated with eight 13C-OA lipids, while males showed few associations. Fetal sex thus influences placental lipid metabolism and could be a key modulator of the impact of maternal metabolic health on perinatal outcomes, potentially contributing toward sex-specific adaptions in which females prioritize survival.
Keywords: diabetes; fatty acid; fetal growth; fetal sex; lipids; placenta; β-oxidation.
Conflict of interest statement
S.-Y.C. and K.M.G. are part of an academic consortium that has received research funding from Abbott Nutrition, Société Des Produits Nestlé S.A., Danone and BenevolentAI Bio Ltd. for work unrelated to this manuscript. S.-Y.C. and K.M.G. are co-inventors on patent filings by Nestlé S.A., which covers the use of inositol in human health applications, but which do not draw on the work in this manuscript. Godfrey has received reimbursement for speaking at conferences sponsored by companies selling nutritional products. Chan has received reimbursement and honoraria into her research funds from Nestlé S.A. for speaking at a conference. The other authors have no financial or personal conflict of interest to declare.
Figures






Similar articles
-
Myo-inositol alters the effects of glucose, leptin and insulin on placental palmitic acid and oleic acid metabolism.J Physiol. 2023 Sep;601(18):4151-4169. doi: 10.1113/JP285036. Epub 2023 Aug 21. J Physiol. 2023. PMID: 37602663 Free PMC article.
-
Placental 13C-DHA metabolism and relationship with maternal BMI, glycemia and birthweight.Mol Med. 2021 Aug 6;27(1):84. doi: 10.1186/s10020-021-00344-w. Mol Med. 2021. PMID: 34362294 Free PMC article.
-
Metabolism of 13C-Labeled Fatty Acids in Term Human Placental Explants by Liquid Chromatography-Mass Spectrometry.Endocrinology. 2019 Jun 1;160(6):1394-1408. doi: 10.1210/en.2018-01020. Endocrinology. 2019. PMID: 30920585
-
Implications of Lipids in Neonatal Body Weight and Fat Mass in Gestational Diabetic Mothers and Non-Diabetic Controls.Curr Diab Rep. 2018 Feb 5;18(2):7. doi: 10.1007/s11892-018-0978-4. Curr Diab Rep. 2018. PMID: 29399727 Review.
-
Placental metabolism and transport of lipid.Fed Proc. 1986 Sep;45(10):2519-23. Fed Proc. 1986. PMID: 3527761 Review.
Cited by
-
AI-based analysis of fetal growth restriction in a prospective obstetric cohort quantifies compound risks for perinatal morbidity and mortality and identifies previously unrecognized high risk clinical scenarios.BMC Pregnancy Childbirth. 2025 Jan 30;25(1):80. doi: 10.1186/s12884-024-07095-6. BMC Pregnancy Childbirth. 2025. PMID: 39881241 Free PMC article.
-
Myo-Inositol Moderates Glucose-Induced Effects on Human Placental 13C-Arachidonic Acid Metabolism.Nutrients. 2022 Sep 26;14(19):3988. doi: 10.3390/nu14193988. Nutrients. 2022. PMID: 36235641 Free PMC article.
-
Increasing maternal age associates with lower placental CPT1B mRNA expression and acylcarnitines, particularly in overweight women.Front Physiol. 2023 May 18;14:1166827. doi: 10.3389/fphys.2023.1166827. eCollection 2023. Front Physiol. 2023. PMID: 37275238 Free PMC article.
-
Sex-specific impact of maternal obesity on fetal placental macrophages and cord blood triglycerides.Placenta. 2023 Sep 7;140:100-108. doi: 10.1016/j.placenta.2023.08.001. Epub 2023 Aug 6. Placenta. 2023. PMID: 37566941 Free PMC article.
-
AI-based analysis of fetal growth restriction in a prospective obstetric cohort quantifies compound risks for perinatal morbidity and mortality and identifies previously unrecognized high risk clinical scenarios.Res Sq [Preprint]. 2024 Dec 16:rs.3.rs-5126218. doi: 10.21203/rs.3.rs-5126218/v1. Res Sq. 2024. Update in: BMC Pregnancy Childbirth. 2025 Jan 30;25(1):80. doi: 10.1186/s12884-024-07095-6. PMID: 39764132 Free PMC article. Updated. Preprint.
References
-
- Voldner N., Frey Frøslie K., Godang K., Bollerslev J., Henriksen T. Determinants of birth weight in boys and girls. Hum. Ontogenet. Int. J. Interdiscip. Dev. Res. 2009;3:7–12. doi: 10.1002/huon.200900001. - DOI
-
- Roland M.C.P., Friis C.M., Godang K., Bollerslev J., Haugen G., Henriksen T. Maternal factors associated with fetal growth and birthweight are independent determinants of placental weight and exhibit differential effects by fetal sex. PLoS ONE. 2014;9:e87303. doi: 10.1371/journal.pone.0087303. - DOI - PMC - PubMed
-
- Group H.S.C.R. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with maternal body mass index. BJOG Int. J. Obstetr. Gynaecol. 2010;117:575–584. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

