On the Computational Design of Azobenzene-Based Multi-State Photoswitches
- PMID: 35955820
- PMCID: PMC9369132
- DOI: 10.3390/ijms23158690
On the Computational Design of Azobenzene-Based Multi-State Photoswitches
Abstract
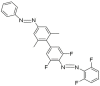
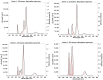
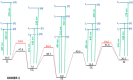
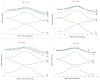
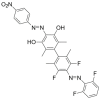
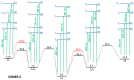
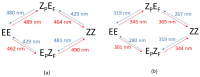
In order to theoretically design multi-state photoswitches with specific properties, an exhaustive computational study is first carried out for an azobenzene dimer that has been recently synthesized and experimentally studied. This study allows for a full comprehension of the factors that govern the photoactivated isomerization processes of these molecules so to provide a conceptual/computational protocol that can be applied to generic multi-state photoswitches. From this knowledge a new dimer with a similar chemical design is designed and also fully characterized. Our theoretical calculations predict that the new dimer proposed is one step further in the quest for a double photoswitch, where the four metastable isomers could be selectively interconverted through the use of different irradiation sequences.
Keywords: absorption spectra; azobenzene dimers; molecular photoswitches; theoretical photochemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Neilson B.M., Bielawski C.W. Illuminating photoswitchable catalysis. ACS Catal. 2013;3:1874–1885. doi: 10.1021/cs4003673. - DOI
-
- Katsonis N., Lubomska M., Pollard M.M., Feringa B.L., Rudolf P. Synthetic light-activated molecular switches and motors on surfaces. Prog. Surf. Sci. 2007;82:407–434. doi: 10.1016/j.progsurf.2007.03.011. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

