Bidirectional Control between Cholesterol Shuttle and Purine Signal at the Central Nervous System
- PMID: 35955821
- PMCID: PMC9369131
- DOI: 10.3390/ijms23158683
Bidirectional Control between Cholesterol Shuttle and Purine Signal at the Central Nervous System
Abstract
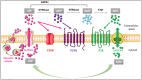
Recent studies have highlighted the mechanisms controlling the formation of cerebral cholesterol, which is synthesized in situ primarily by astrocytes, where it is loaded onto apolipoproteins and delivered to neurons and oligodendrocytes through interactions with specific lipoprotein receptors. The "cholesterol shuttle" is influenced by numerous proteins or carbohydrates, which mainly modulate the lipoprotein receptor activity, function and signaling. These molecules, provided with enzymatic/proteolytic activity leading to the formation of peptide fragments of different sizes and specific sequences, could be also responsible for machinery malfunctions, which are associated with neurological, neurodegenerative and neurodevelopmental disorders. In this context, we have pointed out that purines, ancestral molecules acting as signal molecules and neuromodulators at the central nervous system, can influence the homeostatic machinery of the cerebral cholesterol turnover and vice versa. Evidence gathered so far indicates that purine receptors, mainly the subtypes P2Y2, P2X7 and A2A, are involved in the pathogenesis of neurodegenerative diseases, such as Alzheimer's and Niemann-Pick C diseases, by controlling the brain cholesterol homeostasis; in addition, alterations in cholesterol turnover can hinder the purine receptor function. Although the precise mechanisms of these interactions are currently poorly understood, the results here collected on cholesterol-purine reciprocal control could hopefully promote further research.
Keywords: LDL receptors; cholesterol; purinergic receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Cholesterol homeostasis in neurons and glial cells.Semin Cell Dev Biol. 2005 Apr;16(2):193-212. doi: 10.1016/j.semcdb.2005.01.005. Semin Cell Dev Biol. 2005. PMID: 15797830 Review.
-
Purinoceptor-mediated modulation of purine and neurotransmitter release from nervous tissue.Pharmacol Res. 1998 Mar;37(3):169-78. doi: 10.1006/phrs.1998.0286. Pharmacol Res. 1998. PMID: 9602464 Review.
-
[Cholesterol and Alzheimer's disease].Orv Hetil. 2005 Sep 11;146(37):1903-11. Orv Hetil. 2005. PMID: 16255374 Review. Hungarian.
-
Cholesterol and tau protein--findings in Alzheimer's and Niemann Pick C's disease.Pharmacopsychiatry. 2003 Sep;36 Suppl 2:S120-6. doi: 10.1055/s-2003-43060. Pharmacopsychiatry. 2003. PMID: 14574625
-
Central Nervous System Lipoproteins: ApoE and Regulation of Cholesterol Metabolism.Arterioscler Thromb Vasc Biol. 2016 Jul;36(7):1305-15. doi: 10.1161/ATVBAHA.116.307023. Epub 2016 May 12. Arterioscler Thromb Vasc Biol. 2016. PMID: 27174096 Free PMC article. Review.
Cited by
-
Revealing the Transcriptional and Metabolic Characteristics of Sebocytes Based on the Donkey Cell Transcriptome Atlas.Adv Sci (Weinh). 2025 Apr;12(16):e2413819. doi: 10.1002/advs.202413819. Epub 2025 Feb 27. Adv Sci (Weinh). 2025. PMID: 40013957 Free PMC article.
-
Regulation of synaptic function and lipid metabolism.Neural Regen Res. 2026 Mar 1;21(3):1037-1057. doi: 10.4103/NRR.NRR-D-24-01412. Epub 2025 Apr 29. Neural Regen Res. 2026. PMID: 40313084 Free PMC article.
-
Astroglial Cells: Emerging Therapeutic Targets in the Management of Traumatic Brain Injury.Cells. 2024 Jan 12;13(2):148. doi: 10.3390/cells13020148. Cells. 2024. PMID: 38247839 Free PMC article. Review.
-
Role of Bioactive Molecules on Neuroprotection, Oxidative Stress, and Neuroinflammation Modulation.Int J Mol Sci. 2022 Dec 14;23(24):15925. doi: 10.3390/ijms232415925. Int J Mol Sci. 2022. PMID: 36555565 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

