Four Cholesterol-Recognition Motifs in the Pore-Forming and Translocation Domains of Adenylate Cyclase Toxin Are Essential for Invasion of Eukaryotic Cells and Lysis of Erythrocytes
- PMID: 35955837
- PMCID: PMC9369406
- DOI: 10.3390/ijms23158703
Four Cholesterol-Recognition Motifs in the Pore-Forming and Translocation Domains of Adenylate Cyclase Toxin Are Essential for Invasion of Eukaryotic Cells and Lysis of Erythrocytes
Abstract
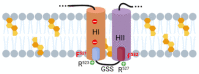
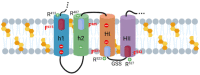
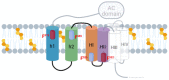
Adenylate Cyclase Toxin (ACT or CyaA) is one of the important virulence factors secreted by Bordetella pertussis, the bacterium causative of whooping cough. ACT debilitates host defenses by production of unregulated levels of cAMP into the cell cytosol upon delivery of its N-terminal domain with adenylate cyclase activity (AC domain) and by forming pores in the plasma membrane of macrophages. Binding of soluble toxin monomers to the plasma membrane of target cells and conversion into membrane-integrated proteins are the first and last step for these toxin activities; however, the molecular determinants in the protein or the target membrane that govern this conversion to an active toxin form are fully unknown. It was previously reported that cytotoxic and cytolytic activities of ACT depend on membrane cholesterol. Here we show that ACT specifically interacts with membrane cholesterol, and find in two membrane-interacting ACT domains, four cholesterol-binding motifs that are essential for AC domain translocation and lytic activities. We hypothesize that direct ACT interaction with membrane cholesterol through those four cholesterol-binding motifs drives insertion and stabilizes the transmembrane topology of several helical elements that ultimately build the ACT structure for AC delivery and pore-formation, thereby explaining the cholesterol-dependence of the ACT activities. The requirement for lipid-mediated stabilization of transmembrane helices appears to be a unifying mechanism to modulate toxicity in pore-forming toxins.
Keywords: RTX toxins; bacterial toxins; lipid-protein interactions; pore-forming toxins.
Conflict of interest statement
The authors declare that they have no conflict of interest with contents of this article. The funding sources had no involvement in the study design nor in the collection, analysis, and interpretation of data nor in the writing of the report or in the decision to submit the article for publication.
Figures
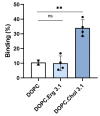
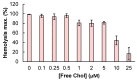
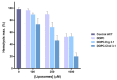
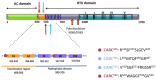
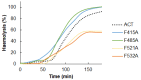
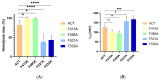

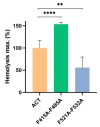
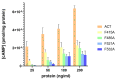
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

