The Roles of Histone Post-Translational Modifications in the Formation and Function of a Mitotic Chromosome
- PMID: 35955838
- PMCID: PMC9368973
- DOI: 10.3390/ijms23158704
The Roles of Histone Post-Translational Modifications in the Formation and Function of a Mitotic Chromosome
Abstract
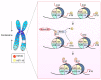
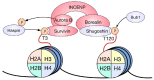
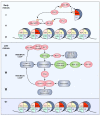
During mitosis, many cellular structures are organized to segregate the replicated genome to the daughter cells. Chromatin is condensed to shape a mitotic chromosome. A multiprotein complex known as kinetochore is organized on a specific region of each chromosome, the centromere, which is defined by the presence of a histone H3 variant called CENP-A. The cytoskeleton is re-arranged to give rise to the mitotic spindle that binds to kinetochores and leads to the movement of chromosomes. How chromatin regulates different activities during mitosis is not well known. The role of histone post-translational modifications (HPTMs) in mitosis has been recently revealed. Specific HPTMs participate in local compaction during chromosome condensation. On the other hand, HPTMs are involved in CENP-A incorporation in the centromere region, an essential activity to maintain centromere identity. HPTMs also participate in the formation of regulatory protein complexes, such as the chromosomal passenger complex (CPC) and the spindle assembly checkpoint (SAC). Finally, we discuss how HPTMs can be modified by environmental factors and the possible consequences on chromosome segregation and genome stability.
Keywords: arsenic; centromere; chromosome condensation; histones; kinetochore; mitosis; nickel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
In vitro centromere and kinetochore assembly on defined chromatin templates.Nature. 2011 Aug 28;477(7364):354-8. doi: 10.1038/nature10379. Nature. 2011. PMID: 21874020 Free PMC article.
-
CENP-A Is Dispensable for Mitotic Centromere Function after Initial Centromere/Kinetochore Assembly.Cell Rep. 2016 Nov 22;17(9):2394-2404. doi: 10.1016/j.celrep.2016.10.084. Cell Rep. 2016. PMID: 27880912 Free PMC article.
-
Centromere Dysfunction Compromises Mitotic Spindle Pole Integrity.Curr Biol. 2019 Sep 23;29(18):3072-3080.e5. doi: 10.1016/j.cub.2019.07.052. Epub 2019 Sep 5. Curr Biol. 2019. PMID: 31495582
-
Critical histone post-translational modifications for centromere function and propagation.Cell Cycle. 2017 Jul 3;16(13):1259-1265. doi: 10.1080/15384101.2017.1325044. Epub 2017 Jun 9. Cell Cycle. 2017. PMID: 28598241 Free PMC article. Review.
-
Plasticity in centromere organization and kinetochore composition: Lessons from diversity.Curr Opin Cell Biol. 2022 Feb;74:47-54. doi: 10.1016/j.ceb.2021.12.007. Epub 2022 Feb 2. Curr Opin Cell Biol. 2022. PMID: 35108654 Free PMC article. Review.
Cited by
-
Compromised Mitotic Fidelity in Human Pluripotent Stem Cells.Int J Mol Sci. 2023 Jul 25;24(15):11933. doi: 10.3390/ijms241511933. Int J Mol Sci. 2023. PMID: 37569309 Free PMC article. Review.
-
Comparative case study on NAMs: towards enhancing specific target organ toxicity analysis.Arch Toxicol. 2024 Nov;98(11):3641-3658. doi: 10.1007/s00204-024-03839-7. Epub 2024 Aug 29. Arch Toxicol. 2024. PMID: 39207506 Free PMC article.
-
Aurora kinases signaling in cancer: from molecular perception to targeted therapies.Mol Cancer. 2025 Jun 18;24(1):180. doi: 10.1186/s12943-025-02353-3. Mol Cancer. 2025. PMID: 40533769 Free PMC article. Review.
-
Liquid Liver Biopsy for Disease Diagnosis and Prognosis.J Clin Transl Hepatol. 2023 Dec 28;11(7):1520-1541. doi: 10.14218/JCTH.2023.00040. Epub 2023 Jul 27. J Clin Transl Hepatol. 2023. PMID: 38161500 Free PMC article. Review.
-
The Role of PARP1 and PAR in ATP-Independent Nucleosome Reorganisation during the DNA Damage Response.Genes (Basel). 2022 Dec 30;14(1):112. doi: 10.3390/genes14010112. Genes (Basel). 2022. PMID: 36672853 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

