Conjugation of the 9-kDa Isoform of Granulysin with Liposomes Potentiates Its Cytotoxicity
- PMID: 35955839
- PMCID: PMC9369117
- DOI: 10.3390/ijms23158705
Conjugation of the 9-kDa Isoform of Granulysin with Liposomes Potentiates Its Cytotoxicity
Abstract
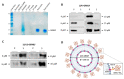
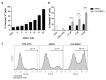
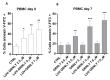
Nine kDa granulysin (GRNLY) is a human cytolytic protein secreted by cytotoxic T lymphocytes (CTL) and NK cells of the immune system whose demonstrated physiological function is the elimination of bacteria and parasites. In previous studies by our group, the anti-tumor capacity of recombinant granulysin was demonstrated, both in vitro and in vivo. In the present work, we developed lipid nanoparticles whose surfaces can bind recombinant granulysin through the formation of a complex of coordination between the histidine tail of the protein and Ni2+ provided by a chelating lipid in the liposome composition and termed them LUV-GRNLY, for granulysin-bound large unilamellar vesicles. The objective of this formulation is to increase the granulysin concentration at the site of contact with the target cell and to increase the cytotoxicity of the administered dose. The results obtained in this work indicate that recombinant granulysin binds to the surface of the liposome with high efficiency and that its cytotoxicity is significantly increased when it is in association with liposomes. In addition, it has been demonstrated that the main mechanism of death induced by both granulysin and LUV-GRNLY is apoptosis. Jurkat-shBak cells are resistant to GRNLY and also to LUV-GRNLY, showing that LUV-GRNLY uses the mitochondrial apoptotic pathway to induce cell death. On the other hand, we show that LUV-GRNLY induces the expression of the pro-apoptotic members of the Bcl-2 family Bim and especially PUMA, although it also induced the expression of anti-apoptotic Bcl-xL. In conclusion, we demonstrate that binding of GRNLY to the surfaces of liposomes clearly augments its cytotoxic potential, with cell death executed mainly by the mitochondrial apoptotic pathway.
Keywords: acute lymphoid leukemia; apoptosis; granulysin; immunotherapy; lipid nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Granulysin induces apoptotic cell death and cleavage of the autophagy regulator Atg5 in human hematological tumors.Biochem Pharmacol. 2014 Feb 1;87(3):410-23. doi: 10.1016/j.bcp.2013.11.004. Epub 2013 Nov 22. Biochem Pharmacol. 2014. PMID: 24269628
-
Immunotherapy with liposome-bound TRAIL overcomes partial protection to soluble TRAIL-induced apoptosis offered by down-regulation of Bim in leukemic cells.Clin Transl Oncol. 2015 Aug;17(8):657-67. doi: 10.1007/s12094-015-1295-x. Epub 2015 May 13. Clin Transl Oncol. 2015. PMID: 25967100
-
Bactericidal and tumoricidal activities of synthetic peptides derived from granulysin.J Immunol. 2000 Aug 1;165(3):1486-90. doi: 10.4049/jimmunol.165.3.1486. J Immunol. 2000. PMID: 10903754
-
Biology and clinical relevance of granulysin.Tissue Antigens. 2009 Mar;73(3):193-8. doi: 10.1111/j.1399-0039.2008.01218.x. Tissue Antigens. 2009. PMID: 19254247 Free PMC article. Review.
-
Granulysin: The attractive side of a natural born killer.Immunol Lett. 2020 Jan;217:126-132. doi: 10.1016/j.imlet.2019.11.005. Epub 2019 Nov 11. Immunol Lett. 2020. PMID: 31726187 Review.
Cited by
-
Disrupting membranes, controlling cell fate: the role of pore-forming proteins in cell death and therapy.Apoptosis. 2025 Jul 21. doi: 10.1007/s10495-025-02133-w. Online ahead of print. Apoptosis. 2025. PMID: 40691738 Review.
-
Nanomedical research and development in Spain: improving the treatment of diseases from the nanoscale.Front Bioeng Biotechnol. 2023 Jul 21;11:1191327. doi: 10.3389/fbioe.2023.1191327. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37545884 Free PMC article. Review.
References
-
- Clayberger C., Finn M.W., Wang T., Saini R., Wilson C., Barr V.A., Sabatino M., Castiello L., Stroncek D., Krensky A.M. 15 kDa Granulysin Causes Differentiation of Monocytes to Dendritic Cells but Lacks Cytotoxic Activity. J. Immunol. 2012;188:6119–6126. doi: 10.4049/jimmunol.1200570. - DOI - PMC - PubMed
-
- Peña S.V., Hanson D.A., Carr B.A., Goralski T.J., Krensky A.M. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. J. Immunol. 1997;158:2680–2688. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

