Molecular Subtyping of Invasive Breast Cancer Using a PAM50-Based Multigene Expression Test-Comparison with Molecular-Like Subtyping by Tumor Grade/Immunohistochemistry and Influence on Oncologist's Decision on Systemic Therapy in a Real-World Setting
- PMID: 35955851
- PMCID: PMC9368794
- DOI: 10.3390/ijms23158716
Molecular Subtyping of Invasive Breast Cancer Using a PAM50-Based Multigene Expression Test-Comparison with Molecular-Like Subtyping by Tumor Grade/Immunohistochemistry and Influence on Oncologist's Decision on Systemic Therapy in a Real-World Setting
Abstract
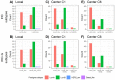
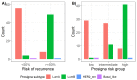
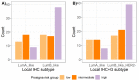
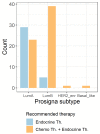
In intermediate risk hormone receptor (HR) positive, HER2 negative breast cancer (BC), the decision regarding adjuvant chemotherapy might be facilitated by multigene expression tests. In all, 142 intermediate risk BCs were investigated using the PAM50-based multigene expression test Prosigna® in a prospective multicentric study. In 119/142 cases, Prosigna® molecular subtyping was compared with local and two central (C1 and C6) molecular-like subtypes relying on both immunohistochemistry (IHC; HRs, HER2, Ki-67) and IHC + tumor grade (IHC+G) subtyping. According to local IHC, 35.4% were Luminal A-like and 64.6% Luminal B-like subtypes (local IHC+G subtype: 31.9% Luminal A-like; 68.1% Luminal B-like). In contrast to local and C1 subtyping, C6 classified >2/3 of cases as Luminal A-like. Pairwise agreement between Prosigna® subtyping and molecular-like subtypes was fair to moderate depending on molecular-like subtyping method and center. The best agreement was observed between Prosigna® (53.8% Luminal A; 44.5% Luminal B) and C1 surrogate subtyping (Cohen’s kappa = 0.455). Adjuvant chemotherapy was suggested to 44.2% and 88.6% of Prosigna® Luminal A and Luminal B cases, respectively. Out of all Luminal A-like cases (locally IHC/IHC+G subtyping), adjuvant chemotherapy was recommended if Prosigna® testing classified as Prosigna® Luminal A at high / intermediate risk or upgraded to Prosigna® Luminal B.
Keywords: IHC; PAM50; Prosigna; breast cancer; chemotherapy; immunohistochemistry; multigene expression analysis; tumor grade.
Conflict of interest statement
P.A.F. has received honoraria from Roche, Pfizer, Novartis, and Celgene. His institution conducts research for Novartis, Cepheid, and BioNTech. A.H. has received honoraria for lectures or consulting/advisory boards for Abbvie, AstraZeneca, Biontech, BMS, Boehringer Ingelheim, Cepheid, Diaceutics, Illumina, Ipsen, Janssen, Lilly, MSD, NanoString, Novartis, Qiagen, QUIP GmbH, Roche, 3DHistotech. R.E. has received honoraria from Roche, Eisai, Pfizer, Mindpeak, AstraZeneca, and Novartis and travel grants from BioNTech. The institution of A.H. and R.E. conducts research for AstraZeneca, Roche, Janssen-Cilag, Zytomed Systems, NanoString Technologies, Veracyte, Biocartis, Novartis, Cepheid, and BioNTech. N.H. reports honoraria for lectures and/or consulting from Amgen, AstraZeneca, Daiichi-Sankyo, Exact Sciences, Lilly, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Sandoz/Hexal, and Seattle Genetics. R.W. served as advisor/consultant/speaker for and/or received travel grants by Agendia, Amgen, Aristo, AstraZeneca, Boeringer Ingelheim, Carl Zeiss, Celgene, Daiichi-Sankyo, Eisai, Exact Sciences, Genomic Health, Gilead, Glaxo Smith Kline, Hexal, Lilly, Medstrom Medical, MSD, Mundipharma, Mylan, Nanostring, Novartis, Odonate, Paxman, Palleos, Pfizer, Pierre Fabre, PumaBiotechnolgogy, Riemser, Roche, Sandoz/Hexal, Sanofi Genzyme, Seattle Genetics/Seagen, Tesaro Bio, Teva, Veracyte, and Viatris. O.H. received honoraria for lectures or consulting/advisory boards for Riemser, Roche, AstraZeneca, Amgen, Pfizer, Eisai, Hexal, MSD, Novartis, SeaGen, and Daiichi-Sankyo. M.P.L. reports honoraria for advisory boards for Lilly, AstraZeneca, MSD, Novartis, Pfizer, Gilead, Eisai, Exact Sciences, Daiichi-Sankyo, Grünenthal, Pierre Fabre, PharmaMar, and Roche; honoraria for lectures for Lilly, Roche, MSD, Novartis, Pfizer, Exact Sciences, Daiichi-Sankyo, Grünenthal, AstraZeneca, and Eisai; editorial board for medac; travel grants for Roche, and Pfizer. The other authors have nothing to declare.
Figures
Similar articles
-
Prospective, multicenter French study evaluating the clinical impact of the Breast Cancer Intrinsic Subtype-Prosigna® Test in the management of early-stage breast cancers.PLoS One. 2017 Oct 18;12(10):e0185753. doi: 10.1371/journal.pone.0185753. eCollection 2017. PLoS One. 2017. PMID: 29045452 Free PMC article.
-
PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers.BMC Med Genomics. 2012 Oct 4;5:44. doi: 10.1186/1755-8794-5-44. BMC Med Genomics. 2012. PMID: 23035882 Free PMC article.
-
The West German Study Group Breast Cancer Intrinsic Subtype study: a prospective multicenter decision impact study utilizing the Prosigna assay for adjuvant treatment decision-making in estrogen-receptor-positive, HER2-negative early-stage breast cancer.Curr Med Res Opin. 2016 Jul;32(7):1217-24. doi: 10.1185/03007995.2016.1166102. Epub 2016 Mar 30. Curr Med Res Opin. 2016. PMID: 26971372
-
Evaluation and Comparison of Prognostic Multigene Tests in Early-Stage Breast Cancer: Which Is the Most Effective? A Literature Review Exploring Clinical Utility to Enhance Therapeutic Management in Luminal Patients.Mol Carcinog. 2025 May;64(5):789-800. doi: 10.1002/mc.23893. Epub 2025 Feb 17. Mol Carcinog. 2025. PMID: 39960127 Free PMC article. Review.
-
Tailoring neoadjuvant treatment of HR-positive/HER2-negative breast cancers: Which role for gene expression assays?Cancer Treat Rev. 2022 Nov;110:102454. doi: 10.1016/j.ctrv.2022.102454. Epub 2022 Aug 11. Cancer Treat Rev. 2022. PMID: 35987149 Review.
Cited by
-
Advancing breast cancer subtyping: optimizing immunohistochemical staining classification with insights from real-world Taiwanese data.Am J Cancer Res. 2023 Nov 15;13(11):5719-5732. eCollection 2023. Am J Cancer Res. 2023. PMID: 38058819 Free PMC article.
-
PROCURE European consensus on breast cancer multigene signatures in early breast cancer management.NPJ Breast Cancer. 2023 Feb 24;9(1):8. doi: 10.1038/s41523-023-00510-9. NPJ Breast Cancer. 2023. PMID: 36828834 Free PMC article.
-
Current Endocrine Therapy in Hormone-Receptor-Positive Breast Cancer: From Tumor Biology to the Rationale for Therapeutic Tunning.Medicina (Kaunas). 2025 Jul 16;61(7):1280. doi: 10.3390/medicina61071280. Medicina (Kaunas). 2025. PMID: 40731911 Free PMC article. Review.
-
The Prediction Analysis of Microarray 50 (PAM50) Gene Expression Classifier Utilized in Indeterminate-Risk Breast Cancer Patients in Hungary: A Consecutive 5-Year Experience.Genes (Basel). 2023 Aug 28;14(9):1708. doi: 10.3390/genes14091708. Genes (Basel). 2023. PMID: 37761848 Free PMC article.
-
The Evolution of Ki-67 and Breast Carcinoma: Past Observations, Present Directions, and Future Considerations.Cancers (Basel). 2023 Jan 28;15(3):808. doi: 10.3390/cancers15030808. Cancers (Basel). 2023. PMID: 36765765 Free PMC article. Review.
References
-
- [(accessed on 27 April 2020)]. Available online: https://www.krebsdaten.de/Krebs/DE/Content/Krebsarten/Brustkrebs/brustkr....
-
- Interdisziplinäre S3-Leitlinie für die Früherkennung, Diagnostik, Therapie und Nachsorge des Mammakarzinoms. Langversion 4. 3 February 2020 AWMF-Registernummer: 032-045OL. [(accessed on 2 August 2022)]. Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/
-
- Arbeitsgemeinschaft Gynäkologische Onkologie e.V. Guidelines Breast Version 2020.1 (Download Gesamtdateien) [(accessed on 26 April 2020)]. Available online: www.ago-online.de.
-
- Allison K.H., Hammond M.E.H., Dowsett M., McKernin S.E., Carey L.A., Fitzgibbons P.L., Hayes D.F., Lakhani S.R., Chavez-MacGregor M., Perlmutter J., et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020;38:1346–1366. doi: 10.1200/JCO.19.02309. - DOI - PubMed
-
- Wolff A.C., Hammond M.E.H., Allison K.H., Harvey B.E., Mangu P.B., Bartlett J.M.S., Bilous M., Ellis I.O., Fitzgibbons P., Hanna W., et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Am. Soc. Clin. Oncol./Coll. Am. Pathol. Clin. Pract. Guidel. Focused Update. 2018;142:1364–1382. doi: 10.5858/arpa.2018-0902-SA. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

