Heritable Epigenomic Modifications Influence Stress Resilience and Rapid Adaptations in the Brown Planthopper (Nilaparvata lugens)
- PMID: 35955860
- PMCID: PMC9368798
- DOI: 10.3390/ijms23158728
Heritable Epigenomic Modifications Influence Stress Resilience and Rapid Adaptations in the Brown Planthopper (Nilaparvata lugens)
Abstract
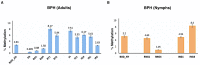
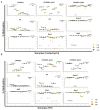
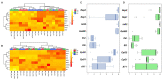
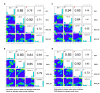
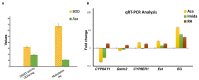
DNA methylation in insects is integral to cellular differentiation, development, gene regulation, genome integrity, and phenotypic plasticity. However, its evolutionary potential and involvement in facilitating rapid adaptations in insects are enigmatic. Moreover, our understanding of these mechanisms is limited to a few insect species, of which none are pests of crops. Hence, we studied methylation patterns in the brown planthopper (BPH), a major rice pest, under pesticide and nutritional stress, across its life stages. Moreover, as the inheritance of epigenetic changes is fundamentally essential for acclimation, adaptability, and evolution, we determined the heritability and persistence of stress-induced methylation marks in BPH across generations. Our results revealed that DNA methylation pattern(s) in BPH varies/vary with environmental cues and is/are insect life-stage specific. Further, our findings provide novel insights into the heritability of stress-induced methylation marks in BPH. However, it was observed that, though heritable, these marks eventually fade in the absence of the stressors, thereby suggesting the existence of fitness cost(s) associated with the maintenance of the stressed epigenotype. Furthermore, we demonstrate how 5-azacytidine-mediated disruption of BPH methylome influences expression levels of stress-responsive genes and, thereby, highlight demethylation/methylation as a phenomenon underlying stress resilience of BPH.
Keywords: DNA methylation; Nilaparvata lugens; adaptive stress response; genetic plasticity; insect epigenetics; plant-insect interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Methylation patterns of Tf2 retrotransposons linked to rapid adaptive stress response in the brown planthopper (Nilaparvata lugens).Genomics. 2021 Nov;113(6):4214-4226. doi: 10.1016/j.ygeno.2021.11.007. Epub 2021 Nov 11. Genomics. 2021. PMID: 34774681
-
Epigenomics in stress tolerance of plants under the climate change.Mol Biol Rep. 2023 Jul;50(7):6201-6216. doi: 10.1007/s11033-023-08539-6. Epub 2023 Jun 9. Mol Biol Rep. 2023. PMID: 37294468 Review.
-
Gene expression and plant hormone levels in two contrasting rice genotypes responding to brown planthopper infestation.BMC Plant Biol. 2017 Feb 28;17(1):57. doi: 10.1186/s12870-017-1005-7. BMC Plant Biol. 2017. PMID: 28245796 Free PMC article.
-
Genetic and molecular understanding of host rice resistance and Nilaparvata lugens adaptation.Curr Opin Insect Sci. 2021 Jun;45:14-20. doi: 10.1016/j.cois.2020.11.005. Epub 2020 Nov 21. Curr Opin Insect Sci. 2021. PMID: 33227482 Review.
-
Molecular basis for insecticide-enhanced thermotolerance in the brown planthopper Nilaparvata lugens Stål (Hemiptera:Delphacidae).Mol Ecol. 2013 Nov;22(22):5624-34. doi: 10.1111/mec.12502. Epub 2013 Oct 16. Mol Ecol. 2013. PMID: 24303791
Cited by
-
Recent Advances in Plant-Insect Interactions.Int J Mol Sci. 2023 Jul 12;24(14):11338. doi: 10.3390/ijms241411338. Int J Mol Sci. 2023. PMID: 37511097 Free PMC article.
-
Unveiling Nilaparvata lugens Stål Genes Defining Compatible and Incompatible Interactions with Rice through Transcriptome Analysis and Gene Silencing.Curr Issues Mol Biol. 2023 Aug 16;45(8):6790-6803. doi: 10.3390/cimb45080429. Curr Issues Mol Biol. 2023. PMID: 37623248 Free PMC article.
-
Epigenetic regulations as drivers of insecticide resistance and resilience to climate change in arthropod pests.Front Genet. 2023 Jan 6;13:1044980. doi: 10.3389/fgene.2022.1044980. eCollection 2022. Front Genet. 2023. PMID: 36685945 Free PMC article. Review.
-
Virulence Adaptation by Rice Planthoppers and Leafhoppers to Resistance Genes and Loci: A Review.Insects. 2024 Aug 29;15(9):652. doi: 10.3390/insects15090652. Insects. 2024. PMID: 39336620 Free PMC article. Review.
-
Examining the Effects of Environment, Geography, and Elevation on Patterns of DNA Methylation Across Populations of Two Widespread Bumble Bee Species.Genome Biol Evol. 2024 Oct 9;16(10):evae207. doi: 10.1093/gbe/evae207. Genome Biol Evol. 2024. PMID: 39327899 Free PMC article.
References
-
- Rodrigues Y.K., Beldade P. Thermal Plasticity in Insects’ Response to Climate Change and to Multifactorial Environments. Front. Ecol. Evol. 2020;8:271. doi: 10.3389/fevo.2020.00271. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

