Cobalt Ferrite Magnetic Nanoparticles for Tracing Mesenchymal Stem Cells in Tissue: A Preliminary Study
- PMID: 35955869
- PMCID: PMC9368918
- DOI: 10.3390/ijms23158738
Cobalt Ferrite Magnetic Nanoparticles for Tracing Mesenchymal Stem Cells in Tissue: A Preliminary Study
Abstract
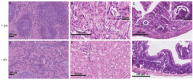
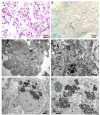
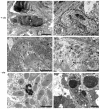
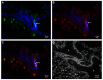
Therapy with mesenchymal stem cells (MSCs) is promising in many diseases. Evaluation of their efficacy depends on adequate follow-up of MSCs after transplantation. Several studies have shown that MSCs can be labeled and subsequently visualized with magnetic nanoparticles (NPs). We investigated the homing of MSCs labeled with magnetic cobalt ferrite NPs in experimentally induced acute kidney injury in mice. To explore the homing of MSCs after systemic infusion into mice, we developed a pre-infusion strategy for optimal tracing and identification of MSCs with polyacrylic acid-coated cobalt ferrite (CoFe2O4) NPs by light and transmission electron microscopy (TEM) in various organs of mice with cisplatin-induced acute kidney injury and control mice. By correlative microscopy, we detected MSCs labeled with NPs in the lungs, spleen, kidney, and intestine of cisplatin-treated mice and in the lungs and spleen of control mice. Our results confirm that labeling MSCs with metal NPs did not affect the ultrastructure of MSCs and their ability to settle in various organs. This study demonstrates the usefulness of cobalt ferrite NPs in ex vivo visualization of MSCs and offers correlative microscopy as a useful method in routine histopathology laboratories for tracing MSCs in paraffin-embedded tissue.
Keywords: markers; mesenchymal stem cells; metal nanoparticles; tissue injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gnecchi M., Danieli P., Malpasso G., Ciuffreda M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Biol. 2016;1416:123–146. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

