Norepinephrine Inhibits Lipopolysaccharide-Stimulated TNF-α but Not Oxylipin Induction in n-3/n-6 PUFA-Enriched Cultures of Circumventricular Organs
- PMID: 35955879
- PMCID: PMC9368774
- DOI: 10.3390/ijms23158745
Norepinephrine Inhibits Lipopolysaccharide-Stimulated TNF-α but Not Oxylipin Induction in n-3/n-6 PUFA-Enriched Cultures of Circumventricular Organs
Abstract
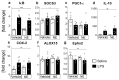
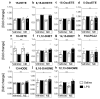
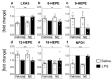
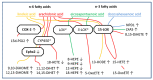
Sensory circumventricular organs (sCVOs) are pivotal brain structures involved in immune-to-brain communication with a leaky blood-brain barrier that detect circulating mediators such as lipopolysaccharide (LPS). Here, we aimed to investigate the potential of sCVOs to produce n-3 and n-6 oxylipins after LPS-stimulation. Moreover, we investigated if norepinephrine (NE) co-treatment can alter cytokine- and oxylipin-release. Thus, we stimulated rat primary neuroglial sCVO cultures under n-3- or n-6-enriched conditions with LPS or saline combined with NE or vehicle. Supernatants were assessed for cytokines by bioassays and oxylipins by HPLC-MS/MS. Expression of signaling pathways and enzymes were analyzed by RT-PCR. Tumor necrosis factor (TNF)α bioactivity and signaling, IL-10 expression, and cyclooxygenase (COX)2 were increased, epoxide hydroxylase (Ephx)2 was reduced, and lipoxygenase 15-(LOX) was not changed by LPS stimulation. Moreover, LPS induced increased levels of several n-6-derived oxylipins, including the COX-2 metabolite 15d-prostaglandin-J2 or the Ephx2 metabolite 14,15-DHET. For n-3-derived oxylipins, some were down- and some were upregulated, including 15-LOX-derived neuroprotectin D1 and 18-HEPE, known for their anti-inflammatory potential. While the LPS-induced increase in TNFα levels was significantly reduced by NE, oxylipins were not significantly altered by NE or changes in TNFα levels. In conclusion, LPS-induced oxylipins may play an important functional role in sCVOs for immune-to-brain communication.
Keywords: circumventricular organs; cytokines; immune-to-brain communication; lipopolysaccharide; oxylipins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Effects of Omega-3 Polyunsaturated Fatty Acids on the Formation of Adipokines, Cytokines, and Oxylipins in Retroperitoneal Adipose Tissue of Mice.Int J Mol Sci. 2024 Sep 13;25(18):9904. doi: 10.3390/ijms25189904. Int J Mol Sci. 2024. PMID: 39337391 Free PMC article.
-
Dietary n-3 long chain PUFA supplementation promotes a pro-resolving oxylipin profile in the brain.Brain Behav Immun. 2019 Feb;76:17-27. doi: 10.1016/j.bbi.2018.07.025. Epub 2018 Aug 4. Brain Behav Immun. 2019. PMID: 30086401
-
Oxylipin Profiles as Functional Characteristics of Acute Inflammatory Responses in Astrocytes Pre-Treated with IL-4, IL-10, or LPS.Int J Mol Sci. 2020 Mar 5;21(5):1780. doi: 10.3390/ijms21051780. Int J Mol Sci. 2020. PMID: 32150861 Free PMC article.
-
Blood Oxylipin Profiles as Markers of Oncological Diseases.Biochemistry (Mosc). 2023 May;88(5):621-629. doi: 10.1134/S000629792305005X. Biochemistry (Mosc). 2023. PMID: 37331708 Review.
-
Effects of omega-3 fatty acid supplementation on the pattern of oxylipins: a short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids.Food Funct. 2017 Jul 19;8(7):2355-2367. doi: 10.1039/c7fo00403f. Food Funct. 2017. PMID: 28682409 Review.
Cited by
-
Effects of Omega-3 Polyunsaturated Fatty Acids on the Formation of Adipokines, Cytokines, and Oxylipins in Retroperitoneal Adipose Tissue of Mice.Int J Mol Sci. 2024 Sep 13;25(18):9904. doi: 10.3390/ijms25189904. Int J Mol Sci. 2024. PMID: 39337391 Free PMC article.
-
Dynamics of oxylipin biosynthesis in systemic inflammation: insights from a large animal model of endotoxemia.Front Immunol. 2025 Jun 16;16:1595888. doi: 10.3389/fimmu.2025.1595888. eCollection 2025. Front Immunol. 2025. PMID: 40589758 Free PMC article.
-
n-3 Polyunsaturated Fatty Acids Modulate LPS-Induced ARDS and the Lung-Brain Axis of Communication in Wild-Type versus Fat-1 Mice Genetically Modified for Leukotriene B4 Receptor 1 or Chemerin Receptor 23 Knockout.Int J Mol Sci. 2023 Aug 31;24(17):13524. doi: 10.3390/ijms241713524. Int J Mol Sci. 2023. PMID: 37686333 Free PMC article.
References
-
- Moranis A., Delpech J.-C., De Smedt-Peyrusse V., Aubert A., Guesnet P., Lavialle M., Joffre C., Layé S. Long term adequate n-3 polyunsaturated fatty acid diet protects from depressive-like behavior but not from working memory disruption and brain cytokine expression in aged mice. Brain Behav. Immun. 2012;26:721–731. doi: 10.1016/j.bbi.2011.11.001. - DOI - PubMed
-
- Sun G.Y., Simonyi A., Fritsche K.L., Chuang D.Y., Hannink M., Gu Z., Greenlief C.M., Yao J.K., Lee J.C., Beversdorf D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fatty Acids. 2018;136:3–13. doi: 10.1016/j.plefa.2017.03.006. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

