Efficacy of Nerve-Derived Hydrogels to Promote Axon Regeneration Is Influenced by the Method of Tissue Decellularization
- PMID: 35955880
- PMCID: PMC9369339
- DOI: 10.3390/ijms23158746
Efficacy of Nerve-Derived Hydrogels to Promote Axon Regeneration Is Influenced by the Method of Tissue Decellularization
Abstract
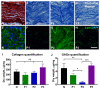
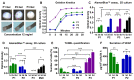
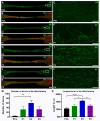
Injuries to large peripheral nerves are often associated with tissue defects and require reconstruction using autologous nerve grafts, which have limited availability and result in donor site morbidity. Peripheral nerve-derived hydrogels could potentially supplement or even replace these grafts. In this study, three decellularization protocols based on the ionic detergents sodium dodecyl sulfate (P1) and sodium deoxycholate (P2), or the organic solvent tri-n-butyl phosphate (P3), were used to prepare hydrogels. All protocols resulted in significantly decreased amounts of genomic DNA, but the P2 hydrogel showed the best preservation of extracellular matrix proteins, cytokines, and chemokines, and reduced levels of sulfated glycosaminoglycans. In vitro P1 and P2 hydrogels supported Schwann cell viability, secretion of VEGF, and neurite outgrowth. Surgical repair of a 10 mm-long rat sciatic nerve gap was performed by implantation of tubular polycaprolactone conduits filled with hydrogels followed by analyses using diffusion tensor imaging and immunostaining for neuronal and glial markers. The results demonstrated that the P2 hydrogel considerably increased the number of axons and the distance of regeneration into the distal nerve stump. In summary, the method used to decellularize nerve tissue affects the efficacy of the resulting hydrogels to support regeneration after nerve injury.
Keywords: MRI; biosynthetic conduit; decellularized nerve tissue; diffusion tensor imaging; nerve-derived hydrogel; peripheral nerve injury.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

