New Advances in Using Virus-like Particles and Related Technologies for Eukaryotic Genome Editing Delivery
- PMID: 35955895
- PMCID: PMC9369418
- DOI: 10.3390/ijms23158750
New Advances in Using Virus-like Particles and Related Technologies for Eukaryotic Genome Editing Delivery
Abstract
The designer nucleases, including Zinc Finger Nuclease (ZFN), Transcription Activator-Like Effector Nuclease (TALEN), and Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated (CRISPR/Cas), have been widely used for mechanistic studies, animal model generation, and gene therapy development. Clinical trials using designer nucleases to treat genetic diseases or cancers are showing promising results. Despite rapid progress, potential off-targets and host immune responses are challenges to be addressed for in vivo uses, especially in clinical applications. Short-term expression of the designer nucleases is necessary to reduce both risks. Currently, delivery methods enabling transient expression of designer nucleases are being pursued. Among these, virus-like particles as delivery vehicles for short-term designer nuclease expression have received much attention. This review will summarize recent developments in using virus-like particles (VLPs) for safe delivery of gene editing effectors to complement our last review on the same topic. First, we introduce some background information on how VLPs can be used for safe and efficient CRISPR/Cas9 delivery. Then, we summarize recently developed virus-like particles as genome editing vehicles. Finally, we discuss applications and future directions.
Keywords: CRISPR/Cas9; RNA; TALEN; ZFN; aptamer; aptamer-binding protein; delivery; designer nuclease; genome editing; ribonucleoprotein; viral capsid; virus-like particle (VLP).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
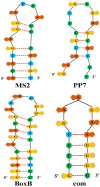
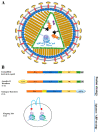
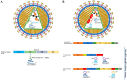
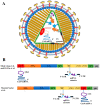
Similar articles
-
Virus-Like Particle Mediated CRISPR/Cas9 Delivery for Efficient and Safe Genome Editing.Life (Basel). 2020 Dec 21;10(12):366. doi: 10.3390/life10120366. Life (Basel). 2020. PMID: 33371215 Free PMC article. Review.
-
Construction and Evaluation of Zinc Finger Nucleases.Methods Mol Biol. 2023;2637:1-25. doi: 10.1007/978-1-0716-3016-7_1. Methods Mol Biol. 2023. PMID: 36773134
-
Genome Editing with mRNA Encoding ZFN, TALEN, and Cas9.Mol Ther. 2019 Apr 10;27(4):735-746. doi: 10.1016/j.ymthe.2019.01.014. Epub 2019 Jan 25. Mol Ther. 2019. PMID: 30803822 Free PMC article. Review.
-
Non-viral delivery of genome-editing nucleases for gene therapy.Gene Ther. 2017 Mar;24(3):144-150. doi: 10.1038/gt.2016.72. Epub 2016 Oct 31. Gene Ther. 2017. PMID: 27797355 Review.
-
Therapeutic Genome Editing and In Vivo Delivery.AAPS J. 2021 Jun 2;23(4):80. doi: 10.1208/s12248-021-00613-w. AAPS J. 2021. PMID: 34080099 Review.
Cited by
-
Viral and nonviral nanocarriers for in vivo CRISPR-based gene editing.Nano Res. 2024 Oct;17(10):8904-8925. doi: 10.1007/s12274-024-6748-5. Epub 2024 Jun 20. Nano Res. 2024. PMID: 40810089 Free PMC article.
-
Significance of VLPs in Vlp-circRNA vaccines: a vaccine candidate or delivery vehicle?RNA Biol. 2024 Jan;21(1):17-28. doi: 10.1080/15476286.2024.2399307. Epub 2024 Sep 6. RNA Biol. 2024. PMID: 39240021 Free PMC article. Review.
-
Multifunctional nanoparticle platform for targeted delivery and vaccines.iScience. 2025 May 7;28(6):112599. doi: 10.1016/j.isci.2025.112599. eCollection 2025 Jun 20. iScience. 2025. PMID: 40496813 Free PMC article.
-
Emerging trends in virus and virus-like particle gene therapy delivery to the brain.Mol Ther Nucleic Acids. 2024 Jul 19;35(3):102280. doi: 10.1016/j.omtn.2024.102280. eCollection 2024 Sep 10. Mol Ther Nucleic Acids. 2024. PMID: 39206077 Free PMC article. Review.
-
Advances in RNA-Based Therapeutics: Challenges and Innovations in RNA Delivery Systems.Curr Issues Mol Biol. 2024 Dec 31;47(1):22. doi: 10.3390/cimb47010022. Curr Issues Mol Biol. 2024. PMID: 39852137 Free PMC article. Review.
References
-
- Nelson C.E., Wu Y., Gemberling M.P., Oliver M.L., Waller M.A., Bohning J.D., Robinson-Hamm J.N., Bulaklak K., Castellanos Rivera R.M., Collier J.H., et al. Long-term evaluation of AAV-CRISPR genome editing for Duchenne muscular dystrophy. Nat. Med. 2019;25:427–432. doi: 10.1038/s41591-019-0344-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

