Multitargeting the Action of 5-HT6 Serotonin Receptor Ligands by Additional Modulation of Kinases in the Search for a New Therapy for Alzheimer's Disease: Can It Work from a Molecular Point of View?
- PMID: 35955902
- PMCID: PMC9368844
- DOI: 10.3390/ijms23158768
Multitargeting the Action of 5-HT6 Serotonin Receptor Ligands by Additional Modulation of Kinases in the Search for a New Therapy for Alzheimer's Disease: Can It Work from a Molecular Point of View?
Abstract
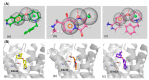
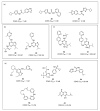
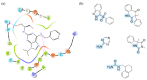
In view of the unsatisfactory treatment of cognitive disorders, in particular Alzheimer's disease (AD), the aim of this review was to perform a computer-aided analysis of the state of the art that will help in the search for innovative polypharmacology-based therapeutic approaches to fight against AD. Apart from 20-year unrenewed cholinesterase- or NMDA-based AD therapy, the hope of effectively treating Alzheimer's disease has been placed on serotonin 5-HT6 receptor (5-HT6R), due to its proven, both for agonists and antagonists, beneficial procognitive effects in animal models; however, research into this treatment has so far not been successfully translated to human patients. Recent lines of evidence strongly emphasize the role of kinases, in particular microtubule affinity-regulating kinase 4 (MARK4), Rho-associated coiled-coil-containing protein kinase I/II (ROCKI/II) and cyclin-dependent kinase 5 (CDK5) in the etiology of AD, pointing to the therapeutic potential of their inhibitors not only against the symptoms, but also the causes of this disease. Thus, finding a drug that acts simultaneously on both 5-HT6R and one of those kinases will provide a potential breakthrough in AD treatment. The pharmacophore- and docking-based comprehensive literature analysis performed herein serves to answer the question of whether the design of these kind of dual agents is possible, and the conclusions turned out to be highly promising.
Keywords: 5-HT6 ligands; Alzheimer’s disease; CDK5; MARK4; ROCKI; ROCKII; dementia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Chemical update on the potential for serotonin 5-HT6 and 5-HT7 receptor agents in the treatment of Alzheimer's disease.Bioorg Med Chem Lett. 2021 Oct 1;49:128275. doi: 10.1016/j.bmcl.2021.128275. Epub 2021 Jul 23. Bioorg Med Chem Lett. 2021. PMID: 34311086 Review.
-
Serotonin 5-HT6 Receptor Antagonists in Alzheimer's Disease: Therapeutic Rationale and Current Development Status.CNS Drugs. 2017 Jan;31(1):19-32. doi: 10.1007/s40263-016-0399-3. CNS Drugs. 2017. PMID: 27914038 Review.
-
Serotonin 5-HT6 receptor antagonists for the treatment of cognitive deficiency in Alzheimer's disease.J Med Chem. 2014 Sep 11;57(17):7160-81. doi: 10.1021/jm5003952. Epub 2014 Jun 3. J Med Chem. 2014. PMID: 24850589 Review.
-
Modulating 5-HT4 and 5-HT6 receptors in Alzheimer's disease treatment.Future Med Chem. 2017 May;9(8):781-795. doi: 10.4155/fmc-2017-0031. Epub 2017 May 15. Future Med Chem. 2017. PMID: 28504917 Review.
-
Novel 1H-Pyrrolo[3,2-c]quinoline Based 5-HT6 Receptor Antagonists with Potential Application for the Treatment of Cognitive Disorders Associated with Alzheimer's Disease.ACS Chem Neurosci. 2016 Jul 20;7(7):972-83. doi: 10.1021/acschemneuro.6b00090. Epub 2016 May 5. ACS Chem Neurosci. 2016. PMID: 27100049
Cited by
-
Anticholinesterase and Serotoninergic Evaluation of Benzimidazole-Carboxamides as Potential Multifunctional Agents for the Treatment of Alzheimer's Disease.Pharmaceutics. 2023 Aug 19;15(8):2159. doi: 10.3390/pharmaceutics15082159. Pharmaceutics. 2023. PMID: 37631373 Free PMC article.
-
5-HT6 receptors: Contemporary views on their neurobiological and pharmacological relevance in neuropsychiatric disorders.Dialogues Clin Neurosci. 2025 Dec;27(1):112-128. doi: 10.1080/19585969.2025.2502028. Epub 2025 May 10. Dialogues Clin Neurosci. 2025. PMID: 40347153 Free PMC article. Review.
References
-
- da Silva E.R., Rodrigues Menezes I.R., Brys I. Effects of Transcranial Direct Current Stimulation on Memory of Elderly People with Mild Cognitive Impairment or Alzheimer’s Disease: A Systematic Review. J. Cent. Nerv. Syst. Dis. 2022;14:117957352211068. doi: 10.1177/11795735221106887. - DOI - PMC - PubMed
-
- García-Casares N., Fuentes P.G., Barbancho M.Á., López-Gigosos R., García-Rodríguez A., Gutiérrez-Bedmar M. Alzheimer’s Disease, Mild Cognitive Impairment and Mediterranean Diet. A Systematic Review and Dose-Response Meta-Analysis. J. Clin. Med. 2021;10:4642. doi: 10.3390/jcm10204642. - DOI - PMC - PubMed
-
- Association A. 2018 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2018;14:367–429. doi: 10.1016/j.jalz.2018.02.001. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

