On the Aggregation of Apolipoprotein A-I
- PMID: 35955915
- PMCID: PMC9369196
- DOI: 10.3390/ijms23158780
On the Aggregation of Apolipoprotein A-I
Abstract
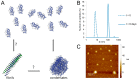
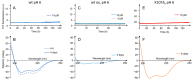
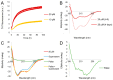
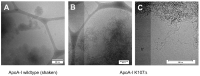
In vivo, apolipoprotein A-I (ApoA-I) is commonly found together with lipids in so-called lipoprotein particles. The protein has also been associated with several diseases-such as atherosclerosis and amyloidosis-where insoluble aggregates containing ApoA-I are deposited in various organs or arteries. The deposited ApoA-I has been found in the form of amyloid fibrils, suggesting that amyloid formation may be involved in the development of these diseases. In the present study we investigated ApoA-I aggregation into amyloid fibrils and other aggregate morphologies. We studied the aggregation of wildtype ApoA-I as well as a disease-associated mutant, ApoA-I K107Δ, under different solution conditions. The aggregation was followed using thioflavin T fluorescence intensity. For selected samples the aggregates formed were characterized in terms of size, secondary structure content, and morphology using circular dichroism spectroscopy, dynamic light scattering, atomic force microscopy and cryo transmission electron microscopy. We find that ApoA-I may form globular protein-only condensates, in which the α-helical conformation of the protein is retained. The protein in its unmodified form appears resistant to amyloid formation; however, the conversion into amyloid fibrils rich in β-sheet is facilitated by oxidation or mutation. In particular, the K107Δ mutant shows higher amyloid formation propensity, and the end state appears to be a co-existence of β-sheet rich amyloid fibrils and α-helix-rich condensates.
Keywords: aggregation; apolipoprotein A-I; condensates; plaques.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Heparin and Methionine Oxidation Promote the Formation of Apolipoprotein A-I Amyloid Comprising α-Helical and β-Sheet Structures.Biochemistry. 2017 Mar 21;56(11):1632-1644. doi: 10.1021/acs.biochem.6b01120. Epub 2017 Mar 13. Biochemistry. 2017. PMID: 27992182
-
Amyloidogenic mutations in human apolipoprotein A-I are not necessarily destabilizing - a common mechanism of apolipoprotein A-I misfolding in familial amyloidosis and atherosclerosis.FEBS J. 2014 Jun;281(11):2525-42. doi: 10.1111/febs.12809. Epub 2014 Apr 28. FEBS J. 2014. PMID: 24702826 Free PMC article.
-
Identification of an amyloid fibril forming peptide comprising residues 46-59 of apolipoprotein A-I.FEBS Lett. 2012 Jun 21;586(13):1754-8. doi: 10.1016/j.febslet.2012.05.007. Epub 2012 May 15. FEBS Lett. 2012. PMID: 22609356
-
The crystal structure of the C-terminal truncated apolipoprotein A-I sheds new light on amyloid formation by the N-terminal fragment.Biochemistry. 2012 Jan 10;51(1):10-8. doi: 10.1021/bi2017014. Epub 2011 Dec 29. Biochemistry. 2012. PMID: 22229410 Free PMC article. Review.
-
β-Barrels and Amyloids: Structural Transitions, Biological Functions, and Pathogenesis.Int J Mol Sci. 2021 Oct 20;22(21):11316. doi: 10.3390/ijms222111316. Int J Mol Sci. 2021. PMID: 34768745 Free PMC article. Review.
Cited by
-
Amyloid, Crohn's disease, and Alzheimer's disease - are they linked?Front Cell Infect Microbiol. 2024 May 8;14:1393809. doi: 10.3389/fcimb.2024.1393809. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38779559 Free PMC article. Review.
-
Studies of All-trans Retinoic Acid Transport in the Eye.Invest Ophthalmol Vis Sci. 2025 Jun 2;66(6):84. doi: 10.1167/iovs.66.6.84. Invest Ophthalmol Vis Sci. 2025. PMID: 40586643 Free PMC article.
-
Physicochemical mechanisms of aggregation and fibril formation of α-synuclein and apolipoprotein A-I.Biophys Physicobiol. 2023 Dec 29;21(1):e210005. doi: 10.2142/biophysico.bppb-v21.0005. eCollection 2024. Biophys Physicobiol. 2023. PMID: 38803339 Free PMC article.
-
Apolipoproteins in Health and Disease.Int J Mol Sci. 2024 Jun 27;25(13):7048. doi: 10.3390/ijms25137048. Int J Mol Sci. 2024. PMID: 39000153 Free PMC article.
-
Anti-inflammatory mechanism of Apolipoprotein A-I.Front Immunol. 2024 Jul 8;15:1417270. doi: 10.3389/fimmu.2024.1417270. eCollection 2024. Front Immunol. 2024. PMID: 39040119 Free PMC article. Review.
References
-
- Westermark P., Benson M.D., Buxbaum J.N., Cohen A.S., Frangione B., Ikeda S.-I., Masters C.L., Merlini G., Saraiva M.J., Sipe J.D. Amyloid: Toward Terminology Clarification. Report from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2005;12:1–4. doi: 10.1080/13506120500032196. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

