Inhibition of the Phospholipase Cε-c-Jun N-Terminal Kinase Axis Suppresses Glioma Stem Cell Properties
- PMID: 35955917
- PMCID: PMC9369372
- DOI: 10.3390/ijms23158785
Inhibition of the Phospholipase Cε-c-Jun N-Terminal Kinase Axis Suppresses Glioma Stem Cell Properties
Abstract
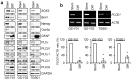
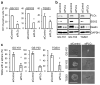
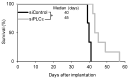
Glioma stem cells (GSCs), the cancer stem cells of glioblastoma multiforme (GBM), contribute to the malignancy of GBM due to their resistance to therapy and tumorigenic potential; therefore, the development of GSC-targeted therapies is urgently needed to improve the poor prognosis of GBM patients. The molecular mechanisms maintaining GSCs need to be elucidated in more detail for the development of GSC-targeted therapy. In comparison with patient-derived GSCs and their differentiated counterparts, we herein demonstrated for the first time that phospholipase C (PLC)ε was highly expressed in GSCs, in contrast to other PLC isoforms. A broad-spectrum PLC inhibitor suppressed the viability of GSCs, but not their stemness. Nevertheless, the knockdown of PLCε suppressed the survival of GSCs and induced cell death. The stem cell capacity of residual viable cells was also suppressed. Moreover, the survival of mice that were transplanted with PLCε knockdown-GSCs was longer than the control group. PLCε maintained the stemness of GSCs via the activation of JNK. The present study demonstrated for the first time that PLCε plays a critical role in maintaining the survival, stemness, and tumor initiation capacity of GSCs. Our study suggested that PLCε is a promising anti-GSC therapeutic target.
Keywords: brain tumor initiating cell; c-Jun N-terminal kinase; glioma initiating cell; phospholipase Cε.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Targeting Folate Metabolism Is Selectively Cytotoxic to Glioma Stem Cells and Effectively Cooperates with Differentiation Therapy to Eliminate Tumor-Initiating Cells in Glioma Xenografts.Int J Mol Sci. 2021 Oct 27;22(21):11633. doi: 10.3390/ijms222111633. Int J Mol Sci. 2021. PMID: 34769063 Free PMC article.
-
Novel function of MDA-9/Syntenin (SDCBP) as a regulator of survival and stemness in glioma stem cells.Oncotarget. 2016 Aug 23;7(34):54102-54119. doi: 10.18632/oncotarget.10851. Oncotarget. 2016. PMID: 27472461 Free PMC article.
-
A new 2-pyrone derivative, 5-bromo-3-(3-hydroxyprop-1-ynyl)-2H-pyran-2-one, suppresses stemness in glioma stem-like cells.Mol Pharmacol. 2012 Sep;82(3):400-7. doi: 10.1124/mol.112.078402. Epub 2012 May 30. Mol Pharmacol. 2012. PMID: 22648970
-
Off the Clock: the Non-canonical Roles of Cyclin-Dependent Kinases in Neural and Glioma Stem Cell Self-Renewal.Mol Neurobiol. 2022 Nov;59(11):6805-6816. doi: 10.1007/s12035-022-03009-9. Epub 2022 Aug 31. Mol Neurobiol. 2022. PMID: 36042143 Review.
-
The Autophagy Status of Cancer Stem Cells in Gliobastoma Multiforme: From Cancer Promotion to Therapeutic Strategies.Int J Mol Sci. 2019 Aug 5;20(15):3824. doi: 10.3390/ijms20153824. Int J Mol Sci. 2019. PMID: 31387280 Free PMC article. Review.
Cited by
-
Phospholipases in Gliomas: Current Knowledge and Future Perspectives from Bench to Bedside.Biomolecules. 2023 May 7;13(5):798. doi: 10.3390/biom13050798. Biomolecules. 2023. PMID: 37238668 Free PMC article. Review.
-
The Novel MDM4 Inhibitor CEP-1347 Activates the p53 Pathway and Blocks Malignant Meningioma Growth In Vitro and In Vivo.Biomedicines. 2023 Jul 12;11(7):1967. doi: 10.3390/biomedicines11071967. Biomedicines. 2023. PMID: 37509605 Free PMC article.
-
Antagonizing MDM2 Overexpression Induced by MDM4 Inhibitor CEP-1347 Effectively Reactivates Wild-Type p53 in Malignant Brain Tumor Cells.Cancers (Basel). 2023 Aug 30;15(17):4326. doi: 10.3390/cancers15174326. Cancers (Basel). 2023. PMID: 37686602 Free PMC article.
References
-
- Sato A., Okada M., Shibuya K., Watanabe E., Seino S., Suzuki K., Narita Y., Shibui S., Kayama T., Kitanaka C. Resveratrol promotes proteasome-dependent degradation of Nanog via p53 activation and induces differentiation of glioma stem cells. Stem Cell Res. 2013;11:601–610. doi: 10.1016/j.scr.2013.04.004. - DOI - PubMed
-
- Sunayama J., Sato A., Matsuda K., Tachibana K., Suzuki K., Narita Y., Shibui S., Sakurada K., Kayama T., Tomiyama A., et al. Dual blocking of mTor and PI3K elicits a prodifferentiation effect on glioblastoma stem-like cells. Neuro-Oncology. 2010;12:1205–1219. doi: 10.1093/neuonc/noq103. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

