Design and Synthesis of Benzene Homologues Tethered with 1,2,4-Triazole and 1,3,4-Thiadiazole Motifs Revealing Dual MCF-7/HepG2 Cytotoxic Activity with Prominent Selectivity via Histone Demethylase LSD1 Inhibitory Effect
- PMID: 35955929
- PMCID: PMC9369007
- DOI: 10.3390/ijms23158796
Design and Synthesis of Benzene Homologues Tethered with 1,2,4-Triazole and 1,3,4-Thiadiazole Motifs Revealing Dual MCF-7/HepG2 Cytotoxic Activity with Prominent Selectivity via Histone Demethylase LSD1 Inhibitory Effect
Abstract
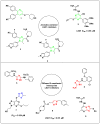
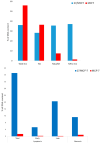
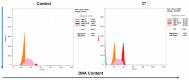
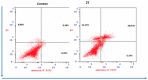
In this study, an efficient multistep synthesis of novel aromatic tricyclic hybrids incorporating different biological active moieties, such as 1,3,4-thiadiazole and 1,2,4-triazole, was reported. These target scaffolds are characterized by having terminal lipophilic or hydrophilic parts, and their structures are confirmed by different spectroscopic methods. Further, the cytotoxic activities of the newly synthesized compounds were evaluated using in vitro MTT cytotoxicity screening assay against three different cell lines, including HepG-2, MCF-7, and HCT-116, compared with the reference drug Taxol. The results showed variable performance against cancer cell lines, exhibiting MCF-7 and HepG-2 selectivities by active analogs. Among these derivatives, 1,2,4-triazoles 11 and 13 and 1,3,4-thiadiazole 18 were found to be the most potent compounds against MCF-7 and HepG-2 cancer cells. Moreover, structure-activity relationship (SAR) studies led to the identification of some potent LSD1 inhibitors. The tested compounds showed good LSD1 inhibitory activities, with an IC50 range of 0.04-1.5 μM. Compounds 27, 23, and 22 were found to be the most active analogs with IC50 values of 0.046, 0.065, and 0.074 μM, respectively. In addition, they exhibited prominent selectivity against a MAO target with apparent cancer cell apoptosis, resulting in DNA fragmentation. This research provides some new aromatic-centered 1,2,4-triazole-3-thione and 1,3,4-thiadiazole analogs as highly effective anticancer agents with good LSD1 target selectivity.
Keywords: 1,2,4-triazoles; 1,3,4-thiadiazoles; LSD1; anticancer activity; apoptosis study; melanoma; tris-substituted analogues.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







References
-
- Garraway L.A., Jänne P.A. Circumventing Cancer Drug Resistance in the Era of Personalized Medicine. Cancer Discov. 2012;2:214–226. doi: 10.1158/2159-8290.CD-12-0012. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

