In Peripheral Blood Mononuclear Cells Helicobacter pylori Induces the Secretion of Soluble and Exosomal Cytokines Related to Carcinogenesis
- PMID: 35955936
- PMCID: PMC9368997
- DOI: 10.3390/ijms23158801
In Peripheral Blood Mononuclear Cells Helicobacter pylori Induces the Secretion of Soluble and Exosomal Cytokines Related to Carcinogenesis
Abstract
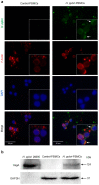
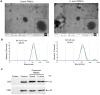
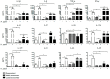
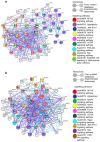
Helicobacter pylori promotes the secretion of cytokines that regulate inflammation and carcinogenesis. Immune cells secrete cytokines into the extracellular medium or packaged in exosomes. The objective of this study was to analyze the profile of soluble and exosomal cytokines that were secreted by human peripheral blood mononuclear cells (PBMCs) that were infected with H. pylori and to build a network of interaction between cytokines and cellular proteins. PBMCs were obtained by density gradient centrifugation and infected with H. pylori for 24 h. The infection was verified by immunofluorescence and Western blot for CagA. The exosomes were obtained from culture supernatant by ultracentrifugation and characterized by transmission electron microscopy, particle size analysis, and Western blot for CD9 and CD81. Cytokines were quantified using a multiplex immunoassay in the culture supernatant, intact exosomes, and lysed exosomes. H. pylori adheres to lymphocytes and translocates CagA. In PBMCs, H. pylori induces an increase in the soluble and exosomal IL-1β, IL-6, TNF-α, IL-10, IL-17A, IL-21, and IL-22. The protein-protein interaction (PPI) network shows that soluble and exosomal cytokines interact with proteins that participate in signaling pathways such as NF-κB, MAPK, PI3K-Akt, Jak-STAT, FoxO, and mTOR, that are related to carcinogenesis; moreover, TNF-α had the highest number of interactions. Cytokine-loaded exosomes represent another means of intercellular communication that is activated by H. pylori to stimulate inflammation, carcinogenesis, or cancer progression. Cytokine-loaded exosomes are likely to be associated with extragastrointestinal diseases of inflammatory origin.
Keywords: Helicobacter pylori; PBMCs; carcinogenesis; cytokines; exosomes; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
α-Lipoic Acid Inhibits Expression of IL-8 by Suppressing Activation of MAPK, Jak/Stat, and NF-κB in H. pylori-Infected Gastric Epithelial AGS Cells.Yonsei Med J. 2016 Jan;57(1):260-4. doi: 10.3349/ymj.2016.57.1.260. Yonsei Med J. 2016. PMID: 26632410 Free PMC article.
-
Exosomal CagA derived from Helicobacter pylori-infected gastric epithelial cells induces macrophage foam cell formation and promotes atherosclerosis.J Mol Cell Cardiol. 2019 Oct;135:40-51. doi: 10.1016/j.yjmcc.2019.07.011. Epub 2019 Jul 25. J Mol Cell Cardiol. 2019. PMID: 31352044
-
Helicobacter pylori Depletes Cholesterol in Gastric Glands to Prevent Interferon Gamma Signaling and Escape the Inflammatory Response.Gastroenterology. 2018 Apr;154(5):1391-1404.e9. doi: 10.1053/j.gastro.2017.12.008. Epub 2017 Dec 19. Gastroenterology. 2018. PMID: 29273450
-
The biological functions of IL-17 in different clinical expressions of Helicobacter pylori-infection.Microb Pathog. 2015 Apr;81:33-8. doi: 10.1016/j.micpath.2015.03.010. Epub 2015 Mar 13. Microb Pathog. 2015. PMID: 25773771 Review.
-
Subversion of host kinases: a key network in cellular signaling hijacked by Helicobacter pylori CagA.Mol Microbiol. 2017 Aug;105(3):358-372. doi: 10.1111/mmi.13707. Epub 2017 May 30. Mol Microbiol. 2017. PMID: 28508421 Review.
Cited by
-
Helicobacter pylori outer membrane vesicles and infected cell exosomes: new players in host immune modulation and pathogenesis.Front Immunol. 2024 Dec 13;15:1512935. doi: 10.3389/fimmu.2024.1512935. eCollection 2024. Front Immunol. 2024. PMID: 39726601 Free PMC article. Review.
-
Extracellular vesicles in Helicobacter pylori-mediated diseases: mechanisms and therapeutic potential.Cell Commun Signal. 2025 Feb 11;23(1):79. doi: 10.1186/s12964-025-02074-6. Cell Commun Signal. 2025. PMID: 39934861 Free PMC article. Review.
-
Gastric microbiome-derived Lacticaseibacillus casei strain RIGLD MG-1 relieves Helicobacter pylori-induced inflammation in gastric epithelial cells in vitro.Mol Biol Rep. 2025 Jul 11;52(1):702. doi: 10.1007/s11033-025-10817-4. Mol Biol Rep. 2025. PMID: 40643767
-
The role of IL-22 in cancer.Med Oncol. 2024 Sep 5;41(10):240. doi: 10.1007/s12032-024-02481-8. Med Oncol. 2024. PMID: 39231878 Review.
-
Immunomodulatory effects of live and pasteurized Lactobacillus crispatus strain RIGLD-1 on Helicobacter pylori-triggered inflammation in gastric epithelial cells in vitro.Mol Biol Rep. 2023 Aug;50(8):6795-6805. doi: 10.1007/s11033-023-08596-x. Epub 2023 Jul 1. Mol Biol Rep. 2023. PMID: 37392285
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

