Novel Antibiofilm Inhibitor Ginkgetin as an Antibacterial Synergist against Escherichia coli
- PMID: 35955943
- PMCID: PMC9369100
- DOI: 10.3390/ijms23158809
Novel Antibiofilm Inhibitor Ginkgetin as an Antibacterial Synergist against Escherichia coli
Abstract
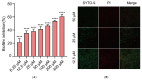
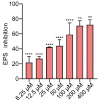
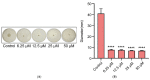
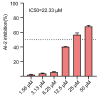
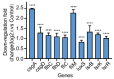
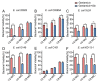
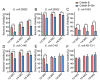
As an opportunistic pathogen, Escherichia coli (E. coli) forms biofilm that increases the virulence of bacteria and antibiotic resistance, posing a serious threat to human and animal health. Recently, ginkgetin (Gin) has been discovered to have antiinflammatory, antioxidant, and antitumor properties. In the present study, we evaluated the antibiofilm and antibacterial synergist of Gin against E. coli. Additionally, Alamar Blue assay combined with confocal laser scanning microscope (CLSM) and crystal violet (CV) staining was used to evaluate the effect of antibiofilm and antibacterial synergist against E. coli. Results showed that Gin reduces biofilm formation, exopolysaccharide (EPS) production, and motility against E. coli without limiting its growth and metabolic activity. Furthermore, we identified the inhibitory effect of Gin on AI-2 signaling molecule production, which showed apparent anti-quorum sensing (QS) properties. The qRT-PCR also indicated that Gin reduced the transcription of curli-related genes (csgA, csgD), flagella-formation genes (flhC, flhD, fliC, fliM), and QS-related genes (luxS, lsrB, lsrK, lsrR). Moreover, Gin showed obvious antibacterial synergism to overcome antibiotic resistance in E. coli with marketed antibiotics, including gentamicin, colistin B, and colistin E. These results suggested the potent antibiofilm and novel antibacterial synergist effect of Gin for treating E. coli infections.
Keywords: EPS; Escherichia coli; antibacterial synergist; antibiofilm; ginkgetin; motility; quorum sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Novel quorum sensing inhibitor Echinatin as an antibacterial synergist against Escherichia coli.Front Microbiol. 2022 Nov 1;13:1003692. doi: 10.3389/fmicb.2022.1003692. eCollection 2022. Front Microbiol. 2022. PMID: 36386683 Free PMC article.
-
Fingolimod Inhibits Exopolysaccharide Production and Regulates Relevant Genes to Eliminate the Biofilm of K. pneumoniae.Int J Mol Sci. 2024 Jan 23;25(3):1397. doi: 10.3390/ijms25031397. Int J Mol Sci. 2024. PMID: 38338675 Free PMC article.
-
Cefoxitin inhibits the formation of biofilm involved in antimicrobial resistance MDR Escherichia coli.Anim Biotechnol. 2025 Dec;36(1):2480176. doi: 10.1080/10495398.2025.2480176. Epub 2025 Mar 23. Anim Biotechnol. 2025. PMID: 40122078
-
Natural and synthetic plant compounds as anti-biofilm agents against Escherichia coli O157:H7 biofilm.Infect Genet Evol. 2021 Nov;95:105055. doi: 10.1016/j.meegid.2021.105055. Epub 2021 Aug 28. Infect Genet Evol. 2021. PMID: 34461310 Review.
-
Identification of novel biofilm genes in avian pathogenic Escherichia coli by Tn5 transposon mutant library.World J Microbiol Biotechnol. 2022 Jun 11;38(8):130. doi: 10.1007/s11274-022-03314-4. World J Microbiol Biotechnol. 2022. PMID: 35688968 Review.
Cited by
-
A novel α-mangostin derivative synergistic to antibiotics against MRSA with unique mechanisms.Microbiol Spectr. 2024 Nov 7;12(12):e0163124. doi: 10.1128/spectrum.01631-24. Online ahead of print. Microbiol Spectr. 2024. PMID: 39508612 Free PMC article.
-
Potential of Flavonoids as Promising Phytotherapeutic Agents to Combat Multidrug-Resistant Infections.Curr Pharm Biotechnol. 2024;25(13):1664-1692. doi: 10.2174/0113892010271172231108190233. Curr Pharm Biotechnol. 2024. PMID: 38031767 Review.
-
Antibacterial activity of camel colostrum against pathogenic strain of Escherichia coli F17-associated with calf diarrhea.Open Vet J. 2024 Nov;14(11):2883-2892. doi: 10.5455/OVJ.2024.v14.i11.17. Epub 2024 Nov 30. Open Vet J. 2024. PMID: 39737040 Free PMC article.
-
Advances and mechanisms of traditional Chinese medicine and its active ingredients against antibiotic-resistant Escherichia coli infections.J Pharm Anal. 2025 Feb;15(2):101117. doi: 10.1016/j.jpha.2024.101117. Epub 2024 Oct 2. J Pharm Anal. 2025. PMID: 40026356 Free PMC article. Review.
-
Polyphenols and CRISPR as Quorum Quenching Agents in Antibiotic-Resistant Foodborne Human Pathogens (Salmonella Typhimurium, Campylobacter jejuni and Escherichia coli 0157:H7).Foods. 2024 Feb 15;13(4):584. doi: 10.3390/foods13040584. Foods. 2024. PMID: 38397561 Free PMC article. Review.
References
-
- Fong J., Yuan M., Jakobsen T.H., Mortensen K.T., Delos Santos M.M., Chua S.L., Yang L., Tan C.H., Nielsen T.E., Givskov M. Disulfide bond-containing ajoene analogues as novel quorum sensing inhibitors of Pseudomonas aeruginosa. J. Med. Chem. 2017;60:215–227. doi: 10.1021/acs.jmedchem.6b01025. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

