The Pnictogen Bond, Together with Other Non-Covalent Interactions, in the Rational Design of One-, Two- and Three-Dimensional Organic-Inorganic Hybrid Metal Halide Perovskite Semiconducting Materials, and Beyond
- PMID: 35955945
- PMCID: PMC9369011
- DOI: 10.3390/ijms23158816
The Pnictogen Bond, Together with Other Non-Covalent Interactions, in the Rational Design of One-, Two- and Three-Dimensional Organic-Inorganic Hybrid Metal Halide Perovskite Semiconducting Materials, and Beyond
Abstract
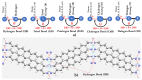
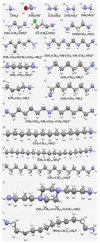
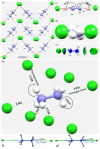
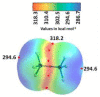
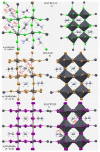
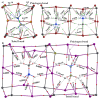
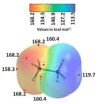
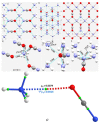
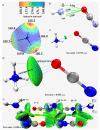
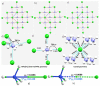
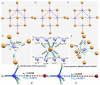
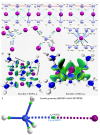
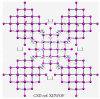
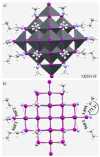
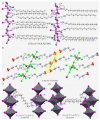
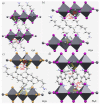
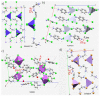
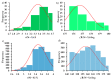
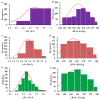
The pnictogen bond, a somewhat overlooked supramolecular chemical synthon known since the middle of the last century, is one of the promising types of non-covalent interactions yet to be fully understood by recognizing and exploiting its properties for the rational design of novel functional materials. Its bonding modes, energy profiles, vibrational structures and charge density topologies, among others, have yet to be comprehensively delineated, both theoretically and experimentally. In this overview, attention is largely centered on the nature of nitrogen-centered pnictogen bonds found in organic-inorganic hybrid metal halide perovskites and closely related structures deposited in the Cambridge Structural Database (CSD) and the Inorganic Chemistry Structural Database (ICSD). Focusing on well-characterized structures, it is shown that it is not merely charge-assisted hydrogen bonds that stabilize the inorganic frameworks, as widely assumed and well-documented, but simultaneously nitrogen-centered pnictogen bonding, and, depending on the atomic constituents of the organic cation, other non-covalent interactions such as halogen bonding and/or tetrel bonding, are also contributors to the stabilizing of a variety of materials in the solid state. We have shown that competition between pnictogen bonding and other interactions plays an important role in determining the tilting of the MX6 (X = a halogen) octahedra of metal halide perovskites in one, two and three-dimensions. The pnictogen interactions are identified to be directional even in zero-dimensional crystals, a structural feature in many engineered ordered materials; hence an interplay between them and other non-covalent interactions drives the structure and the functional properties of perovskite materials and enabling their application in, for example, photovoltaics and optoelectronics. We have demonstrated that nitrogen in ammonium and its derivatives in many chemical systems acts as a pnictogen bond donor and contributes to conferring stability, and hence functionality, to crystalline perovskite systems. The significance of these non-covalent interactions should not be overlooked, especially when the focus is centered on the rationale design and discovery of such highly-valued materials.
Keywords: ICSD and CSD database analyses; IGM-δg analysis; MESP characterizations; inorganic-organic hybrid halide perovskites; intermolecular geometries and directionality; nitrogen as pnictogen bond donor; pnictogen bonding; sum of the van der Waals radii concept.
Conflict of interest statement
The authors declare no conflict of interest. The funders had absolutely no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

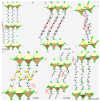

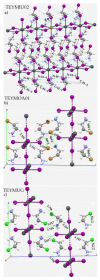
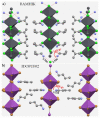
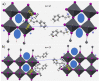





References
-
- Foster S.L., Bakovic S.I.P., Duda R.D., Maheshwari S., Milton R.D., Minteer S.D., Janik M.J., Renner J.N., Greenlee L.F. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 2018;1:490–500. doi: 10.1038/s41929-018-0092-7. - DOI
-
- Uyanik M., Kato T., Sahara N., Katade O., Ishihara K. High-Performance Ammonium Hypoiodite/Oxone Catalysis for Enantioselective Oxidative Dearomatization of Arenols. ACS Catal. 2019;9:11619–11626. doi: 10.1021/acscatal.9b04322. - DOI
-
- Hinokuma S., Sato K. Ammonia Combustion Catalysts. Chem. Lett. 2021;50:752–759. doi: 10.1246/cl.200843. - DOI
-
- Lamb K.E., Dolan M.D., Kennedy D.F. Ammonia for hydrogen storage; A review of catalytic ammonia decomposition and hydrogen separation and purification. Int. J. Hydrogen Energy. 2019;44:3580–3593. doi: 10.1016/j.ijhydene.2018.12.024. - DOI
-
- US Geological Survey . Mineral Commodity Summaries 2020. US Geological Survey; Reston, VA, USA: 2020. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

