Accurate Determination of the Degree of Deacetylation of Chitosan Using UPLC-MS/MS
- PMID: 35955947
- PMCID: PMC9369293
- DOI: 10.3390/ijms23158810
Accurate Determination of the Degree of Deacetylation of Chitosan Using UPLC-MS/MS
Abstract
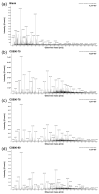
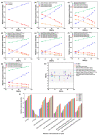
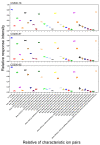
The mole fraction of deacetylated monomeric units in chitosan (CS) molecules is referred to as CS's degree of deacetylation (DD). In this study, 35 characteristic ions of CS were detected using liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS/MS). The relative response intensity of 35 characteristic ion pairs using a single charge in nine CS samples with varying DDs was analyzed using 30 analytical methods. There was a good linear relationship between the relative response intensity of the characteristic ion pairs determined using ultrahigh performance (UP) LC-MS/MS and the DD of CS. The UPLC-MS/MS method for determining the DD of CS was unaffected by the sample concentration. The detection instrument has a wide range of application parameters with different voltages, high temperatures, and gas flow conditions. This study established a detection method for the DD of CS with high sensitivity, fast analysis, accuracy, stability, and durability.
Keywords: UPLC–MS/MS; chitosan; degree of deacetylation determination; relative response intensity of the characteristic peak.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Development of a mass spectrometry method for the characterization of a series of chitosan.Int J Biol Macromol. 2019 Jan;121:89-96. doi: 10.1016/j.ijbiomac.2018.09.194. Epub 2018 Sep 30. Int J Biol Macromol. 2019. PMID: 30282015
-
A novel method to quantify chitosan in aqueous solutions by ultrahigh-performance liquid chromatography-tandem mass spectrometry.Carbohydr Polym. 2024 Apr 1;329:121758. doi: 10.1016/j.carbpol.2023.121758. Epub 2024 Jan 2. Carbohydr Polym. 2024. PMID: 38286539
-
Determination of colistin A and colistin B in human plasma by UPLC-ESI high resolution tandem MS: application to a pharmacokinetic study.J Pharm Biomed Anal. 2013 Sep;83:228-36. doi: 10.1016/j.jpba.2013.05.008. Epub 2013 May 16. J Pharm Biomed Anal. 2013. PMID: 23764659
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
-
Derivatization in liquid chromatography for mass spectrometric detection.Drug Discov Ther. 2013 Feb;7(1):9-17. doi: 10.5582/ddt.2013.v7.1.9. Drug Discov Ther. 2013. PMID: 23524938 Review.
Cited by
-
Anti-Candida and Anti-Leishmanial Activities of Encapsulated Cinnamomum verum Essential Oil in Chitosan Nanoparticles.Molecules. 2023 Jul 27;28(15):5681. doi: 10.3390/molecules28155681. Molecules. 2023. PMID: 37570651 Free PMC article.
-
Double-Network Chitosan-Based Hydrogels with Improved Mechanical, Conductive, Antimicrobial, and Antibiofouling Properties.Gels. 2023 Mar 29;9(4):278. doi: 10.3390/gels9040278. Gels. 2023. PMID: 37102890 Free PMC article. Review.
-
Application of chitosan in fruit preservation: A review.Food Chem X. 2024 Jun 22;23:101589. doi: 10.1016/j.fochx.2024.101589. eCollection 2024 Oct 30. Food Chem X. 2024. PMID: 39036472 Free PMC article. Review.
-
Chitosan: Structural and Chemical Modification, Properties, and Application.Int J Mol Sci. 2023 Dec 31;25(1):554. doi: 10.3390/ijms25010554. Int J Mol Sci. 2023. PMID: 38203726 Free PMC article.
References
-
- Ravi Kumar M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000;46:1–27. doi: 10.1016/S1381-5148(00)00038-9. - DOI
-
- Domard A. A perspective on 30 years research on chitin and chitosan. Carbohydr. Polym. 2011;84:696–703. doi: 10.1016/j.carbpol.2010.04.083. - DOI
-
- Pillai C.K.S., Paul W., Sharma C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009;34:641–678. doi: 10.1016/j.progpolymsci.2009.04.001. - DOI
-
- Muzzarelli R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009;76:167–182. doi: 10.1016/j.carbpol.2008.11.002. - DOI
-
- Rinaudo M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006;31:603–632. doi: 10.1016/j.progpolymsci.2006.06.001. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

