LEAF TIP RUMPLED 1 Regulates Leaf Morphology and Salt Tolerance in Rice
- PMID: 35955949
- PMCID: PMC9369171
- DOI: 10.3390/ijms23158818
LEAF TIP RUMPLED 1 Regulates Leaf Morphology and Salt Tolerance in Rice
Abstract
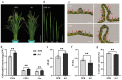
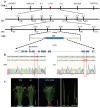
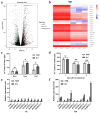
Leaf morphology is one of the important traits related to ideal plant architecture and is an important factor determining rice stress resistance, which directly affects yield. Wax layers form a barrier to protect plants from different environmental stresses. However, the regulatory effect of wax synthesis genes on leaf morphology and salt tolerance is not well-understood. In this study, we identified a rice mutant, leaf tip rumpled 1 (ltr1), in a mutant library of the classic japonica variety Nipponbare. Phenotypic investigation of NPB and ltr1 suggested that ltr1 showed rumpled leaf with uneven distribution of bulliform cells and sclerenchyma cells, and disordered vascular bundles. A decrease in seed-setting rate in ltr1 led to decreased per-plant grain yield. Moreover, ltr1 was sensitive to salt stress, and LTR1 was strongly induced by salt stress. Map-based cloning of LTR1 showed that there was a 2-bp deletion in the eighth exon of LOC_Os02g40784 in ltr1, resulting in a frameshift mutation and early termination of transcription. Subsequently, the candidate gene was confirmed using complementation, overexpression, and knockout analysis of LOC_Os02g40784. Functional analysis of LTR1 showed that it was a wax synthesis gene and constitutively expressed in entire tissues with higher relative expression level in leaves and panicles. Moreover, overexpression of LTR1 enhanced yield in rice and LTR1 positively regulates salt stress by affecting water and ion homeostasis. These results lay a theoretical foundation for exploring the molecular mechanism of leaf morphogenesis and stress response, providing a new potential strategy for stress-tolerance breeding.
Keywords: Oryza sativa L.; aquaporin; bulliform cells; leaf shape; salt stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

