Albumin Stimulates Epithelial Na+ Transport and Barrier Integrity by Activating the PI3K/AKT/SGK1 Pathway
- PMID: 35955955
- PMCID: PMC9368928
- DOI: 10.3390/ijms23158823
Albumin Stimulates Epithelial Na+ Transport and Barrier Integrity by Activating the PI3K/AKT/SGK1 Pathway
Abstract
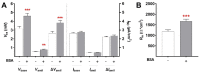
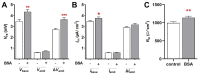
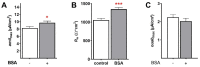
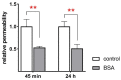
Albumin is a major serum protein and is frequently used as a cell culture supplement. It is crucially involved in the regulation of osmotic pressure and distribution of fluid between different compartments. Alveolar epithelial Na+ transport drives alveolar fluid clearance (AFC), enabling air breathing. Whether or not albumin affects AFC and Na+ transport is yet unknown. We therefore determined the acute and chronic effects of albumin on Na+ transport in fetal distal lung epithelial (FDLE) cells and the involved kinase pathways. Chronic BSA treatment strongly increased epithelial Na+ transport and barrier integrity in Ussing chambers. BSA did not elevate mRNA expression of Na+ transporters in FDLE cells after 24 h. Moreover, acute BSA treatment for 45 min mimicked the chronic effects. The elevated Na+ transport was caused by an increased maximal ENaC activity, while Na,K-ATPase activity remained unchanged. Acute and chronic BSA treatment lowered membrane permeability, confirming the increased barrier integrity observed in Ussing chambers. Western blots demonstrated an increased phosphorylation of AKT and SGK1, and PI3K inhibition abolished the stimulating effect of BSA. BSA therefore enhanced epithelial Na+ transport and barrier integrity by activating the PI3K/AKT/SGK1 pathway.
Keywords: BSA; ENaC; Na+ transport; barrier integrity; lung; protein kinase B.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Maresin1 stimulates alveolar fluid clearance through the alveolar epithelial sodium channel Na,K-ATPase via the ALX/PI3K/Nedd4-2 pathway.Lab Invest. 2017 May;97(5):543-554. doi: 10.1038/labinvest.2016.150. Epub 2017 Feb 20. Lab Invest. 2017. PMID: 28218740
-
Epidermal growth factor strongly affects epithelial Na+ transport and barrier function in fetal alveolar cells, with minor sex-specific effects.Sci Rep. 2021 Aug 5;11(1):15951. doi: 10.1038/s41598-021-95410-y. Sci Rep. 2021. PMID: 34354180 Free PMC article.
-
Regulation of ENaC-mediated alveolar fluid clearance by insulin via PI3K/Akt pathway in LPS-induced acute lung injury.Respir Res. 2012 Mar 30;13(1):29. doi: 10.1186/1465-9921-13-29. Respir Res. 2012. PMID: 22458687 Free PMC article.
-
SGK1 regulation of epithelial sodium transport.Cell Physiol Biochem. 2003;13(1):13-20. doi: 10.1159/000070245. Cell Physiol Biochem. 2003. PMID: 12649598 Review.
-
SGK1: aldosterone-induced relay of Na+ transport regulation in distal kidney nephron cells.Cell Physiol Biochem. 2003;13(1):21-8. doi: 10.1159/000070246. Cell Physiol Biochem. 2003. PMID: 12649599 Review.
Cited by
-
Developing an interpretable machine learning predictive model of chronic obstructive pulmonary disease by serum PFAS concentration.Front Public Health. 2025 Jul 10;13:1602566. doi: 10.3389/fpubh.2025.1602566. eCollection 2025. Front Public Health. 2025. PMID: 40709045 Free PMC article.
-
Serum/glucocorticoid regulated kinase 1 (SGK1) in neurological disorders: pain or gain.Exp Neurol. 2024 Dec;382:114973. doi: 10.1016/j.expneurol.2024.114973. Epub 2024 Sep 24. Exp Neurol. 2024. PMID: 39326820 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

