Stellettin B-Induced Oral Cancer Cell Death via Endoplasmic Reticulum Stress-Mitochondrial Apoptotic and Autophagic Signaling Pathway
- PMID: 35955957
- PMCID: PMC9368952
- DOI: 10.3390/ijms23158813
Stellettin B-Induced Oral Cancer Cell Death via Endoplasmic Reticulum Stress-Mitochondrial Apoptotic and Autophagic Signaling Pathway
Abstract
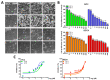
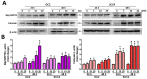
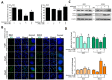
Oral squamous cell carcinoma (OSCC) affects tens of thousands of people worldwide. Despite advances in cancer treatment, the 5-year survival rate of patients with late-stage OSCC is low at 50-60%. Therefore, the development of anti-OSCC therapy is necessary. We evaluated the effects of marine-derived triterpene stellettin B in human OC2 and SCC4 cells. Stellettin B dose-dependently decreased the viability of both cell lines, with a significant reduction in OC2 cells at ≥0.1 µM at 24 and 48 h, and in SCC4 cells at ≥1 µM at 24 and 48 h. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-positive cells were significantly observed at 20 µM of stellettin B at 48 h, with the overexpression of cleaved caspase3 and cleaved poly(ADP-ribose) polymerase (PARP). Moreover, mitochondrial respiratory functions were ablated by stellettin B. Autophagy-related LC3-II/LC3-I ratio and Beclin-1 proteins were increased, whereas p62 was decreased. At 20 µM at 48 h, the expression levels of the endoplasmic reticulum (ER) stress biomarkers calnexin and BiP/GRP78 were significantly increased and mitogen-activated protein kinase (MAPK) signaling pathways were activated. Further investigation using the autophagy inhibitor 3-methyladenine (3-MA) demonstrated that it alleviated stellettin B-induced cell death and autophagy. Overall, our findings show that stellettin B induces the ER stress, mitochondrial stress, apoptosis, and autophagy, causing cell death of OSCC cells.
Keywords: BiP/GRP78; ER stress; autophagy; mitochondrial stress; stellettin B.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Isomahanine induces endoplasmic reticulum stress and simultaneously triggers p38 MAPK-mediated apoptosis and autophagy in multidrug-resistant human oral squamous cell carcinoma cells.Oncol Rep. 2017 Feb;37(2):1243-1252. doi: 10.3892/or.2017.5352. Epub 2017 Jan 4. Oncol Rep. 2017. PMID: 28075474
-
Naringenin induces endoplasmic reticulum stress-mediated cell apoptosis and autophagy in human oral squamous cell carcinoma cells.J Food Biochem. 2022 Nov;46(11):e14221. doi: 10.1111/jfbc.14221. Epub 2022 May 21. J Food Biochem. 2022. PMID: 35596593
-
Cantharidin Induced Oral Squamous Cell Carcinoma Cell Apoptosis via the JNK-Regulated Mitochondria and Endoplasmic Reticulum Stress-Related Signaling Pathways.PLoS One. 2016 Dec 8;11(12):e0168095. doi: 10.1371/journal.pone.0168095. eCollection 2016. PLoS One. 2016. PMID: 27930712 Free PMC article.
-
Deficiency in the mitochondrial apoptotic pathway reveals the toxic potential of autophagy under ER stress conditions.Autophagy. 2014;10(11):1921-36. doi: 10.4161/15548627.2014.981790. Autophagy. 2014. PMID: 25470234 Free PMC article.
-
Autophagy and apoptosis in liver injury.Cell Cycle. 2015;14(11):1631-42. doi: 10.1080/15384101.2015.1038685. Cell Cycle. 2015. PMID: 25927598 Free PMC article. Review.
Cited by
-
Autophagy in oral cancer: Promises and challenges (Review).Int J Mol Med. 2024 Dec;54(6):116. doi: 10.3892/ijmm.2024.5440. Epub 2024 Oct 18. Int J Mol Med. 2024. PMID: 39422076 Free PMC article. Review.
-
Pituitary tumor‑transforming gene 1 regulates the senescence and apoptosis of oral squamous cell carcinoma in a p21‑dependent DNA damage response manner.Oncol Rep. 2024 Oct;52(4):135. doi: 10.3892/or.2024.8794. Epub 2024 Aug 19. Oncol Rep. 2024. PMID: 39155881 Free PMC article.
-
Stellettin B Induces Cell Death in Bladder Cancer Via Activating the Autophagy/DAPK2/Apoptosis Signaling Cascade.Mar Drugs. 2023 Jan 21;21(2):73. doi: 10.3390/md21020073. Mar Drugs. 2023. PMID: 36827114 Free PMC article.
-
Targeting the mTOR Pathway in Hepatocellular Carcinoma: The Therapeutic Potential of Natural Products.J Inflamm Res. 2024 Dec 6;17:10421-10440. doi: 10.2147/JIR.S501270. eCollection 2024. J Inflamm Res. 2024. PMID: 39659752 Free PMC article. Review.
-
Knockdown of Y-box binding protein 1 induces autophagy in early porcine embryos.Front Cell Dev Biol. 2023 Oct 30;11:1238546. doi: 10.3389/fcell.2023.1238546. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37965572 Free PMC article.
References
-
- Ko C.Y., Shih P.C., Huang P.W., Lee Y.H., Chen Y.F., Tai M.H., Liu C.H., Wen Z.H., Kuo H.M. Sinularin, an anti-cancer agent causing mitochondria-modulated apoptosis and cytoskeleton disruption in human hepatocellular carcinoma. Int. J. Mol. Sci. 2021;22:3946. doi: 10.3390/ijms22083946. - DOI - PMC - PubMed
-
- Yuan C.H., Ma Y.L., Shih P.C., Chen C.T., Cheng S.Y., Pan C.Y., Jean Y.H., Chu Y.M., Lin S.C., Lai Y.C., et al. The antimicrobial peptide tilapia piscidin 3 induces mitochondria-modulated intrinsic apoptosis of osteosarcoma cells. Biochem. Pharmacol. 2020;178:114064–114075. doi: 10.1016/j.bcp.2020.114064. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

