Forest Biomass as a Promising Source of Bioactive Essential Oil and Phenolic Compounds for Alzheimer's Disease Therapy
- PMID: 35955963
- PMCID: PMC9369093
- DOI: 10.3390/ijms23158812
Forest Biomass as a Promising Source of Bioactive Essential Oil and Phenolic Compounds for Alzheimer's Disease Therapy
Abstract
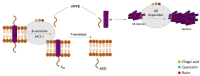
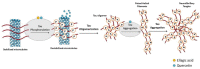
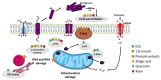
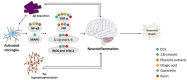
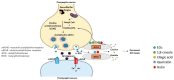
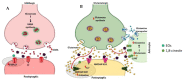
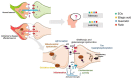
Alzheimer's disease (AD) is the most common neurodegenerative disorder affecting elderly people worldwide. Currently, there are no effective treatments for AD able to prevent disease progression, highlighting the urgency of finding new therapeutic strategies to stop or delay this pathology. Several plants exhibit potential as source of safe and multi-target new therapeutic molecules for AD treatment. Meanwhile, Eucalyptus globulus extracts revealed important pharmacological activities, namely antioxidant and anti-inflammatory properties, which can contribute to the reported neuroprotective effects. This review summarizes the chemical composition of essential oil (EO) and phenolic extracts obtained from Eucalyptus globulus leaves, disclosing major compounds and their effects on AD-relevant pathological features, including deposition of amyloid-β (Aβ) in senile plaques and hyperphosphorylated tau in neurofibrillary tangles (NFTs), abnormalities in GABAergic, cholinergic and glutamatergic neurotransmission, inflammation, and oxidative stress. In general, 1,8-cineole is the major compound identified in EO, and ellagic acid, quercetin, and rutin were described as main compounds in phenolic extracts from Eucalyptus globulus leaves. EO and phenolic extracts, and especially their major compounds, were found to prevent several pathological cellular processes and to improve cognitive function in AD animal models. Therefore, Eucalyptus globulus leaves are a relevant source of biological active and safe molecules that could be used as raw material for nutraceuticals and plant-based medicinal products useful for AD prevention and treatment.
Keywords: Alzheimer’s disease; essential oil; eucalyptus; phenolic extracts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Effect of bioactive extracts from Eucalyptus globulus leaves in experimental models of Alzheimer's disease.Biomed Pharmacother. 2024 Dec;181:117652. doi: 10.1016/j.biopha.2024.117652. Epub 2024 Oct 31. Biomed Pharmacother. 2024. PMID: 39486370
-
Chemical Composition and Effect against Skin Alterations of Bioactive Extracts Obtained by the Hydrodistillation of Eucalyptus globulus Leaves.Pharmaceutics. 2022 Mar 3;14(3):561. doi: 10.3390/pharmaceutics14030561. Pharmaceutics. 2022. PMID: 35335937 Free PMC article.
-
Extra-Virgin Olive Oil in Alzheimer's Disease: A Comprehensive Review of Cellular, Animal, and Clinical Studies.Int J Mol Sci. 2024 Feb 5;25(3):1914. doi: 10.3390/ijms25031914. Int J Mol Sci. 2024. PMID: 38339193 Free PMC article. Review.
-
Antioxidant activity, neuroprotective properties and bioactive constituents analysis of varying polarity extracts from Eucalyptus globulus leaves.J Food Drug Anal. 2018 Oct;26(4):1293-1302. doi: 10.1016/j.jfda.2018.05.010. Epub 2018 Jun 18. J Food Drug Anal. 2018. PMID: 30249328 Free PMC article.
-
Therapeutic potentials of plant iridoids in Alzheimer's and Parkinson's diseases: A review.Eur J Med Chem. 2019 May 1;169:185-199. doi: 10.1016/j.ejmech.2019.03.009. Epub 2019 Mar 8. Eur J Med Chem. 2019. PMID: 30877973 Review.
Cited by
-
Exploring the mechanism of agarwood moxa smoke in treating sleep disorders based on GC-MS and network pharmacology.Front Med (Lausanne). 2024 May 9;11:1400334. doi: 10.3389/fmed.2024.1400334. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38784223 Free PMC article.
References
-
- Patterson C. World Alzheimer Report 2018: The State of the Art of Dementia Research: New Frontiers. Alzheimer’s Disease International (ADI); London, UK: 2018.
-
- Braak H., de Vos R.A., Jansen E.N., Bratzke H., Braak E. Neuropathological hallmarks of Alzheimer’s and Parkinson’s diseases. Prog. Brain. Res. 1998;117:267–285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

