Proadrenomedullin in the Management of COVID-19 Critically Ill Patients in Intensive Care Unit: A Systematic Review and Meta-Analysis of Evidence and Uncertainties in Existing Literature
- PMID: 35956159
- PMCID: PMC9369672
- DOI: 10.3390/jcm11154543
Proadrenomedullin in the Management of COVID-19 Critically Ill Patients in Intensive Care Unit: A Systematic Review and Meta-Analysis of Evidence and Uncertainties in Existing Literature
Abstract
Mid-regional proadrenomedullin (MR-proADM) is a new biomarker of endothelial damage and its clinical use is increasing in sepsis and respiratory infections and recently in SARS-CoV-2 infection. We conducted a systematic review and meta-analysis to clarify the use of MR-proADM in severe COVID-19 disease. After Pubmed, Embase, and Scopus search, registries, and gray literature, deduplication, and selection of full-texts, we found 21 studies addressing the use of proadrenomedullin in COVID-19. All the studies were published between 2020 and 2022 from European countries. A total of 9 studies enrolled Intensive Care Unit (ICU) patients, 4 were conducted in the Emergency Department, and 8 had mixed populations. Regarding the ICU critically ill patients, 4 studies evaluating survival as primary outcome were available, of which 3 reported completed data. Combining the selected studies in a meta-analysis, a total of 252 patients were enrolled; of these, 182 were survivors and 70 were non-survivors. At the admission to the ICU, the average MR-proADM level in survivor patients was 1.01 versus 1.64 in non-survivor patients. The mean differences of MR-proADM values in survivors vs. non-survivors was −0.96 (95% CI from −1.26, to −0.65). Test for overall effect: Z = 6.19 (p < 0.00001) and heterogeneity was I2 = 0%. MR-proADM ICU admission levels seem to predict mortality among the critical COVID-19 population. Further, prospective studies, focused on critically ill patients and investigating a reliable MR-proADM cut-off, are needed to provide adequate guidance to its use in severe COVID-19.
Keywords: COVID-19; MR-proADM; SARS-CoV-2; biomarkers; endothelitis; intensive care; proadrenomedullin.
Conflict of interest statement
All the authors declare that they have no conflict of interest in the field of the present article.
Figures

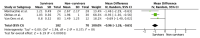
References
-
- Saeed K., Legramante J.M., Angeletti S., Curcio F., Miguens I., Poole S., Tascini C., Sozio E., Del Castillo J.G. Mid-regional pro-adrenomedullin as a supplementary tool to clinical parameters in cases of suspicion of infection in the emergency department. Expert Rev. Mol. Diagn. 2021;21:397–404. doi: 10.1080/14737159.2021.1902312. - DOI - PubMed
-
- Saeed K., Wilson D.C., Bloos F., Schuetz P., van der Does Y., Melander O., Hausfater P., Legramante J.M., Claessens Y.-E., Amin D., et al. The early identification of disease progression in patients with suspected infection presenting to the emergency department: A multi-centre derivation and validation study. Crit. Care. 2019;23:40. doi: 10.1186/s13054-019-2329-5. - DOI - PMC - PubMed
-
- Elke G., Bloos F., Wilson D.C., Brunkhorst F.M., Briegel J., Reinhart K., Loeffler M., Kluge S., Nierhaus A., Jaschinski U., et al. The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis-a secondary analysis of a large randomised controlled trial. Crit. Care. 2018;22:79. doi: 10.1186/s13054-018-2001-5. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

