Replacement of Valproic Acid with New Anti-Seizure Medications in Idiopathic Generalized Epilepsy
- PMID: 35956197
- PMCID: PMC9369717
- DOI: 10.3390/jcm11154582
Replacement of Valproic Acid with New Anti-Seizure Medications in Idiopathic Generalized Epilepsy
Abstract
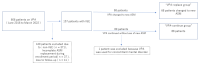
Background: Little is known regarding the non-inferiority of new anti-seizure medications (ASMs) in terms of replacing valproic acid (VPA) in patients with idiopathic generalized epilepsy (IGE). We hypothesized that replacement of VPA with new ASMs would offer non-inferior or better control of seizure frequency. The purpose of this study was to compare epileptic seizure frequency between the subset of patients with IGE who were on VPA and the subset of patients with IGE who replaced VPA with new ASMs. Methods: Patients with IGE who were on or had been on VPA between January 2016 and March 2022 were divided into a group that replaced VPA with new ASMs (VPA-replace group) and a group that remained on VPA (VPA-continue group). We then compared the groups in terms of seizure frequency and myoclonus. Results: Of the 606 patients on VPA between January 2016 and March 2022, 156 patients with IGE were enrolled to this study (VPA-replace group, n = 68; VPA-continue group, n = 88). The VPA-replace group included significantly more females than the VPA-continue group (p < 0.001). The VPA-replace group also showed significantly higher seizure frequency before replacement (p < 0.001), but not after replacement (p = 0.074). Patients on monotherapy displayed improved seizure frequency with new ASMs (p < 0.001). Among the new ASMs, perampanel (PER) significantly improved seizure frequency (p = 0.002). Forty-two patients in the VPA-replace group who had myoclonus achieved significant improvements (p < 0.001). Among these, patients on PER monotherapy (p < 0.001) or PER + lamotrigine (0.016) showed significantly improved myoclonus scale scores. Conclusions: This study shows the non-inferiority of new ASMs compared to VPA, with better seizure control using new ASMs in subsets of patients with IGE on monotherapy.
Keywords: anti-seizure medication (ASM); idiopathic generalized epilepsy (IGE); myoclonus; replacement; seizure frequency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Kanner A.M., Ashman E., Gloss D., Harden C., Bourgeois B., Bautista J.F., Abou-Khalil B., Burakgazi-Dalkilic E., Llanas Park E., Stern J., et al. Practice guideline update summary: Efficacy and tolerability of the new antiepileptic drugs II: Treatment-resistant epilepsy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2018;91:82–90. doi: 10.1212/wnl.0000000000005756. - DOI - PubMed
LinkOut - more resources
Full Text Sources

