Hesperidin Improves Memory Function by Enhancing Neurogenesis in a Mouse Model of Alzheimer's Disease
- PMID: 35956303
- PMCID: PMC9370591
- DOI: 10.3390/nu14153125
Hesperidin Improves Memory Function by Enhancing Neurogenesis in a Mouse Model of Alzheimer's Disease
Abstract
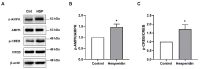
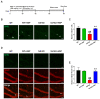
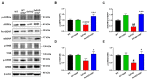
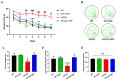
Alzheimer's disease (AD) is an irreversible neurodegenerative disease characterized by memory and cognitive impairments. Neurogenesis, which is related to memory and cognitive function, is reduced in the brains of patients with AD. Therefore, enhancing neurogenesis is a potential therapeutic strategy for neurodegenerative diseases, including AD. Hesperidin (HSP), a bioflavonoid found primarily in citrus plants, has anti-inflammatory, antioxidant, and neuroprotective effects. The objective of this study was to determine the effects of HSP on neurogenesis in neural stem cells (NSCs) isolated from the brain of mouse embryos and five familial AD (5xFAD) mice. In NSCs, HSP significantly increased the proliferation of NSCs by activating adenosine monophosphate (AMP)-activated protein kinase (AMPK)/cAMP-response element-binding protein (CREB) signaling, but did not affect NSC differentiation into neurons and astrocytes. HSP administration restored neurogenesis in the hippocampus of 5xFAD mice via AMPK/brain-derived neurotrophic factor/tropomyosin receptor kinase B/CREB signaling, thereby decreasing amyloid-beta accumulation and ameliorating memory dysfunction. Collectively, these preclinical findings suggest that HSP is a promising candidate for the prevention and treatment of AD.
Keywords: Alzheimer’s disease; hesperidin; neural stem cells; neurogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Blurton-Jones M., Kitazawa M., Martinez-Coria H., Castello N.A., Müller F.-J., Loring J.F., Yamasaki T.R., Poon W.W., Green K.N., LaFerla F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. - DOI - PMC - PubMed
-
- Moreno-Jimenez E.P., Flor-Garcia M., Terreros-Roncal J., Rabano A., Cafini F., Pallas-Bazarra N., Avila J., Llorens-Martin M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

