The Circadian Regulation of Nutrient Metabolism in Diet-Induced Obesity and Metabolic Disease
- PMID: 35956312
- PMCID: PMC9370226
- DOI: 10.3390/nu14153136
The Circadian Regulation of Nutrient Metabolism in Diet-Induced Obesity and Metabolic Disease
Abstract
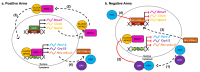
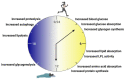
Obesity and other metabolic diseases are major public health issues that are particularly prevalent in industrialized societies where circadian rhythmicity is disturbed by shift work, jet lag, and/or social obligations. In mammals, daylight entrains the hypothalamic suprachiasmatic nucleus (SCN) to a ≈24 h cycle by initiating a transcription/translation feedback loop (TTFL) of molecular clock genes. The downstream impacts of the TTFL on clock-controlled genes allow the SCN to set the rhythm for the majority of physiological, metabolic, and behavioral processes. The TTFL, however, is ubiquitous and oscillates in tissues throughout the body. Tissues outside of the SCN are entrained to other signals, such as fed/fasting state, rather than light input. This system requires a considerable amount of biological flexibility as it functions to maintain homeostasis across varying conditions contained within a 24 h day. In the face of either circadian disruption (e.g., jet lag and shift work) or an obesity-induced decrease in metabolic flexibility, this finely tuned mechanism breaks down. Indeed, both human and rodent studies have found that obesity and metabolic disease develop when endogenous circadian pacing is at odds with the external cues. In the following review, we will delve into what is known on the circadian rhythmicity of nutrient metabolism and discuss obesity as a circadian disease.
Keywords: circadian rhythms; metabolism; molecular clock; obesity.
Conflict of interest statement
M.A.L. is an advisory board member and has received research support from Pfizer Inc., advisory board member and co-founder of Flare Therapeutics, and consultant to Madrigal.
Figures

References
-
- Twig G., Reichman B., Afek A., Derazne E., Hamiel U., Furer A., Gershovitz L., Bader T., Cukierman-Yaffe T., Kark J.D., et al. Severe Obesity and Cardio-Metabolic Comorbidities: A Nationwide Study of 2.8 Million Adolescents. Int. J. Obes. 2018;437:1391–1399. doi: 10.1038/s41366-018-0213-z. - DOI - PubMed
-
- Aghili S.M.M., Ebrahimpur M., Arjmand B., Shadman Z., Pejman Sani M., Qorbani M., Larijani B., Payab M. Obesity in COVID-19 Era, Implications for Mechanisms, Comorbidities, and Prognosis: A Review and Meta-Analysis. Int. J. Obes. 2021;45:998–1016. doi: 10.1038/s41366-021-00776-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

