Inflammatory Signatures of Maternal Obesity as Risk Factors for Neurodevelopmental Disorders: Role of Maternal Microbiota and Nutritional Intervention Strategies
- PMID: 35956326
- PMCID: PMC9370669
- DOI: 10.3390/nu14153150
Inflammatory Signatures of Maternal Obesity as Risk Factors for Neurodevelopmental Disorders: Role of Maternal Microbiota and Nutritional Intervention Strategies
Abstract
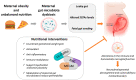
Obesity is a main risk factor for the onset and the precipitation of many non-communicable diseases. This condition, which is associated with low-grade chronic systemic inflammation, is of main concern during pregnancy leading to very serious consequences for the new generations. In addition to the prominent role played by the adipose tissue, dysbiosis of the maternal gut may also sustain the obesity-related inflammatory milieu contributing to create an overall suboptimal intrauterine environment. Such a condition here generically defined as "inflamed womb" may hold long-term detrimental effects on fetal brain development, increasing the vulnerability to mental disorders. In this review, we will examine the hypothesis that maternal obesity-related gut dysbiosis and the associated inflammation might specifically target fetal brain microglia, the resident brain immune macrophages, altering neurodevelopmental trajectories in a sex-dependent fashion. We will also review some of the most promising nutritional strategies capable to prevent or counteract the effects of maternal obesity through the modulation of inflammation and oxidative stress or by targeting the maternal microbiota.
Keywords: gut microbiota; high-fat diet; inflammation; maternal obesity; microglia; nutritional intervention strategies; oxidative stress.
Conflict of interest statement
A.B., F.C. and C.M. are currently running experimental activities dealing with the administration of MFGM (provided by Reckitt|Mead Johnson Nutrition Institute) in animal models. M.A.A.-C. and R.D.S. declare no conflict of interest.
Figures
References
-
- Reemst K., Tims S., Yam K.-Y., Mischke M., Knol J., Brul S., Schipper L., Korosi A. The Role of the Gut Microbiota in the Effects of Early-Life Stress and Dietary Fatty Acids on Later-Life Central and Metabolic Outcomes in Mice. mSystems. 2022;7:e00180-22. doi: 10.1128/msystems.00180-22. - DOI - PMC - PubMed
-
- Gomes D., Le L., Perschbacher S., Haas N.A., Netz H., Hasbargen U., Delius M., Lange K., Nennstiel U., Roscher A.A., et al. Predicting the earliest deviation in weight gain in the course towards manifest overweight in offspring exposed to obesity in pregnancy: A longitudinal cohort study. BMC Med. 2022;20:1–18. doi: 10.1186/s12916-022-02318-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

