Effect of Early and Intensive Telephone or Electronic Nutrition Counselling Delivered to People with Upper Gastrointestinal Cancer on Quality of Life: A Three-Arm Randomised Controlled Trial
- PMID: 35956410
- PMCID: PMC9370208
- DOI: 10.3390/nu14153234
Effect of Early and Intensive Telephone or Electronic Nutrition Counselling Delivered to People with Upper Gastrointestinal Cancer on Quality of Life: A Three-Arm Randomised Controlled Trial
Abstract
Background: Delay in dietetic service provision for upper gastrointestinal cancer exacerbates disease-related malnutrition and consequently increases morbidity and mortality. Dietetic services are usually referral-based and provided face-to-face in inpatient or outpatient settings, which can delay the commencement of nutrition care. The aim of this study was to provide intensive dietetic intervention close to the time of diagnosis for upper gastrointestinal cancer and assess the effect on quality-adjusted life years.
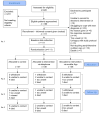
Methods: A three-arm randomised controlled trial of adults newly diagnosed with upper gastrointestinal cancer was performed. A behavioural-based, individually tailored, symptom-directed nutrition intervention was provided in addition to usual care, delivered by a dietitian using a telephone (synchronously) or a mobile application (asynchronously) for 18 weeks, compared with a usual care control group. Data were collected at baseline, three, six, and twelve months post-randomisation. The primary outcome was quality-adjusted life years (EQ-5D-5L quality of life assessment tool). Data were analysed using linear mixed models.
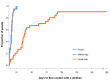
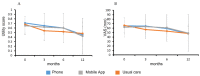
Results: One hundred and eleven participants were randomised. Quality-adjusted life years were not different in the intervention groups compared with control (telephone: mean (95% CI) 0.04 (0.43, 2.3), p = 0.998; App: -0.08 (-0.18, 0.02), p = 0.135) after adjustment for baseline, nutrition risk status, age, and gender. Survival was similar between groups over 12 months. The asynchronous mobile app group had a greater number of withdrawals compared with the telephone group.
Conclusion: Early and intensive nutrition counselling, delivered at home, during anticancer treatment did not change quality-adjusted life years or survival over 12 months compared with usual care. Behavioural counselling alone was unable to achieve nutritional adequacy. Dietetic services delivered asynchronously using a mobile app had low acceptance for patients undergoing anticancer treatment.
Trial registration: 27 January 2017 Australian and New Zealand Clinical Trial Registry, ACTRN12617000152325.
Keywords: behaviour change; dietetic intervention; mHealth; malnutrition; quality-adjusted life years; upper gastrointestinal cancer.
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form. L.H., K.F., M.-A.S., J.S., D.C., P.C. and L.L. were all employees of the Health Service that was the predominant site of recruitment. D.C., P.C. and L.L. referred patients with a histological diagnosis of included cancers, but were not involved with screening, obtaining informed consent, or randomisation. T.H. has provided expert witness testimony on the topic of the prevention of falls in hospitals for Minter Ellison Law Firm and K&L Gates Law Firm within the past 36 months.
Figures
References
-
- Guo Z.Q., Yu J.M., Li W., Fu Z.M., Lin Y., Shi Y.Y., Hu W., Ba Y., Li S.Y., Li Z.N., et al. Survey and analysis of the nutritional status in hospitalized patients with malignant gastric tumors and its influence on the quality of life. Supportive Care Cancer Off. J. Multinatl. Assoc. Supportive Care Cancer. 2020;28:373–380. doi: 10.1007/s00520-019-04803-3. - DOI - PMC - PubMed
-
- Dewys W.D., Begg C., Lavin P.T., Band P.R., Bennett J.M., Bertino J.R., Cohen M.H., Douglass H.O., Jr., Engstrom P.F., Ezdinli E.Z., et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am. J. Med. 1980;69:491–497. doi: 10.1016/S0149-2918(05)80001-3. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

