Effects of Probiotics on Gut Microbiomes of Extremely Preterm Infants in the Neonatal Intensive Care Unit: A Prospective Cohort Study
- PMID: 35956415
- PMCID: PMC9370381
- DOI: 10.3390/nu14153239
Effects of Probiotics on Gut Microbiomes of Extremely Preterm Infants in the Neonatal Intensive Care Unit: A Prospective Cohort Study
Abstract
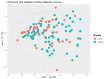
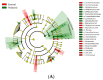
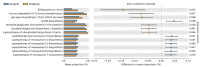
Background: Probiotics have been previously reported to reduce the incidence of necrotizing enterocolitis (NEC) in extremely preterm infants, but the mechanisms by which the probiotics work remain unknown. We aimed to investigate the effects of probiotics on the gut microbiota of extremely preterm infants. Methods: A prospective cohort study was conducted on 120 extremely preterm neonates (gestational age ≤ 28 weeks) between August 2019 and December 2021. All neonates were divided into the study (receiving probiotics) and the control (no probiotics) groups. Multivariate logistic regression analysis was performed to investigate the significantly different compositions of gut microbiota between these two groups. The effects of probiotics on the occurrence of NEC and late-onset sepsis were also investigated. Results: An increased abundance of Lactobacillus was noted in neonates who received the probiotics (AOR 4.33; 95% CI, 1.89-9.96, p = 0.009) when compared with the control group. Subjects in the probiotic group had significantly fewer days of total parenteral nutrition (median [interquartile range, IQR]) 29.0 (26.8-35.0) versus 35.5 (27.8-45.0), p = 0.004) than those in the control group. The probiotic group had a significantly lower rate of late-onset sepsis than the control group (47.1% versus 70.0%, p = 0.015), but the rate of NEC, duration of hospitalization and the final in-hospital mortality rates were comparable between these two groups. Conclusions: Probiotic supplementation of extremely preterm infants soon after the initiation of feeding increased the abundance of Lactobacillus. Probiotics may reduce the risk of late-onset sepsis, but further randomized controlled trials are warranted in the future.
Keywords: gut microbiome; necrotizing enterocolitis; neonatal immunity; neonates; probiotics.
Conflict of interest statement
All authors declare no conflict of interest in this study.
Figures



Similar articles
-
Maternal probiotic supplementation for prevention of morbidity and mortality in preterm infants.Cochrane Database Syst Rev. 2018 Dec 12;12(12):CD012519. doi: 10.1002/14651858.CD012519.pub2. Cochrane Database Syst Rev. 2018. PMID: 30548483 Free PMC article.
-
Probiotics to prevent necrotising enterocolitis and nosocomial infection in very low birth weight preterm infants.Br J Nutr. 2017 Apr;117(7):994-1000. doi: 10.1017/S0007114517000769. Epub 2017 Apr 26. Br J Nutr. 2017. PMID: 28443531
-
Impact of Clinical Use of Probiotics on Preterm-Related Outcomes in Infants with Extremely Low Birth Weight.Nutrients. 2024 Sep 5;16(17):2995. doi: 10.3390/nu16172995. Nutrients. 2024. PMID: 39275310 Free PMC article.
-
Probiotics for prevention of necrotizing enterocolitis in preterm infants.Evid Based Child Health. 2014 Sep;9(3):584-671. doi: 10.1002/ebch.1976. Evid Based Child Health. 2014. PMID: 25236307 Review.
-
Probiotic supplementation and risk of necrotizing enterocolitis and mortality among extremely preterm infants-the Probiotics in Extreme Prematurity in Scandinavia (PEPS) trial: study protocol for a multicenter, double-blinded, placebo-controlled, and registry-based randomized controlled trial.Trials. 2024 Apr 12;25(1):259. doi: 10.1186/s13063-024-08088-8. Trials. 2024. PMID: 38610034 Free PMC article.
Cited by
-
Age disparities in intestinal stem cell quantities: a possible explanation for preterm infant susceptibility to necrotizing enterocolitis.Pediatr Surg Int. 2022 Dec;38(12):1971-1979. doi: 10.1007/s00383-022-05257-1. Epub 2022 Oct 8. Pediatr Surg Int. 2022. PMID: 36208323
-
Therapeutic Potential of Gut Microbiota and Its Metabolite Short-Chain Fatty Acids in Neonatal Necrotizing Enterocolitis.Life (Basel). 2023 Feb 16;13(2):561. doi: 10.3390/life13020561. Life (Basel). 2023. PMID: 36836917 Free PMC article. Review.
-
Gram-Negative Colonization and Bacterial Translocation Drive Neonatal Sepsis in the Indian Setting.J Epidemiol Glob Health. 2024 Dec;14(4):1525-1535. doi: 10.1007/s44197-024-00303-8. Epub 2024 Sep 30. J Epidemiol Glob Health. 2024. PMID: 39347930 Free PMC article.
-
Probiotic Supplements Effect on Feeding Tolerance, Growth and Neonatal Morbidity in Extremely Preterm Infants: A Systematic Review and Meta-Analysis.Nutrients. 2025 Apr 1;17(7):1228. doi: 10.3390/nu17071228. Nutrients. 2025. PMID: 40218986 Free PMC article.
-
Emerging issues in probiotic safety: 2023 perspectives.Gut Microbes. 2023 Jan-Dec;15(1):2185034. doi: 10.1080/19490976.2023.2185034. Gut Microbes. 2023. PMID: 36919522 Free PMC article.
References
-
- Fujimura K.E., Sitarik A.R., Havstad S., Lin D.L., LeVan S., Fadrosh D., Panzer A.R., LaMere B., Rackaityte E., Lukacs N.W., et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat. Med. 2016;22:1187–1191. doi: 10.1038/nm.4176. - DOI - PMC - PubMed
-
- Cukrowska B., Bierła J.B., Zakrzewska M., Klukowski M., Maciorkowska E. The Relationship between the Infant Gut Microbiota and Allergy. The Role of Bifidobacterium breve and Prebiotic Oligosaccharides in the Activation of Anti-Allergic Mechanisms in Early Life. Nutrients. 2020;12:946. doi: 10.3390/nu12040946. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

