The Distance between Minima of Electron Density and Electrostatic Potential as a Measure of Halogen Bond Strength
- PMID: 35956799
- PMCID: PMC9369751
- DOI: 10.3390/molecules27154848
The Distance between Minima of Electron Density and Electrostatic Potential as a Measure of Halogen Bond Strength
Abstract
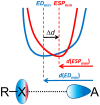
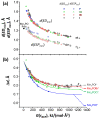
In this study, we present results of a detailed topological analysis of electron density (ED) of 145 halogen-bonded complexes formed by various fluorine-, chlorine-, bromine-, and iodine-containing compounds with trimethylphosphine oxide, Me3PO. To characterize the halogen bond (XB) strength, we used the complexation enthalpy, the interatomic distance between oxygen and halogen, as well as the typical set of electron density properties at the bond critical points calculated at B3LYP/jorge-ATZP level of theory. We show for the first time that it is possible to predict the XB strength based on the distance between the minima of ED and molecular electrostatic potential (ESP) along the XB path. The gap between ED and ESP minima exponentially depends on local electronic kinetic energy density at the bond critical point and tends to be a common limiting value for the strongest halogen bond.
Keywords: 31P NMR; QTAIM; bond strength; density functional theory; electron density; electrostatic potential; halogen bond; interaction energy; phosphine oxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

