Modulation of High-Fat Diet-Induced Brain Oxidative Stress by Ferulate-Rich Germinated Brown Rice Ethyl Acetate Extract
- PMID: 35956857
- PMCID: PMC9369880
- DOI: 10.3390/molecules27154907
Modulation of High-Fat Diet-Induced Brain Oxidative Stress by Ferulate-Rich Germinated Brown Rice Ethyl Acetate Extract
Abstract
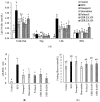
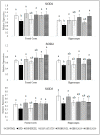
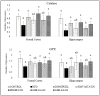
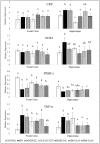
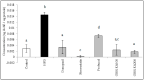
The oxidative stress resulting from the production of reactive oxygen species plays a vital role in inflammatory processes and is associated with neurodegenerative changes. In view of the ability of germinated brown rice (GBR) to improve learning and memory, this present study aimed to investigate the mechanistic basis of GBR's neuroprotection in a high-fat diet (HFD)-induced oxidative changes in adult Sprague-Dawley rats. Ferulate-rich GBR ethyl acetate extract (GBR-EA; 100 mg/kg and 200 mg/kg body weight) was supplemented orally for the last 3 months of 6 months HFD feeding during the study. GBR-EA supplementation was found to improve lipid profile and serum antioxidant status, when compared to the HFD group. Elevated mRNA expressions of SOD1, SOD2, SOD3, Catalase, and GPX were demonstrated in the frontal cortex and hippocampus of GBR-EA treated animals. The pro-inflammatory changes induced by HFD in the hippocampus were attenuated by GBR-EA through the downregulation of CRP and TNF- α and upregulation of PPAR-γ. GBR also reduced the hippocampal mRNA expression and enzyme level of acetylcholinesterase. In conclusion, this study proposed the possible transcriptomic regulation of antioxidant and inflammation in neurodegenerative processes resulting from high cholesterol consumption, with an emphasis on GBR's potential to ameliorate such changes.
Keywords: Alzheimer’s disease; acetylcholinesterase; brain oxidative stress; germinated brown rice; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Ethyl acetate extract of germinated brown rice attenuates hydrogen peroxide-induced oxidative stress in human SH-SY5Y neuroblastoma cells: role of anti-apoptotic, pro-survival and antioxidant genes.BMC Complement Altern Med. 2013 Jul 17;13:177. doi: 10.1186/1472-6882-13-177. BMC Complement Altern Med. 2013. PMID: 23866310 Free PMC article.
-
In utero exposure to germinated brown rice and its oryzanol-rich extract attenuated high fat diet-induced insulin resistance in F1 generation of rats.BMC Complement Altern Med. 2017 Jan 21;17(1):67. doi: 10.1186/s12906-017-1571-0. BMC Complement Altern Med. 2017. PMID: 28109299 Free PMC article.
-
Anti-obesity effects of germinated brown rice extract through down-regulation of lipogenic genes in high fat diet-induced obese mice.Biosci Biotechnol Biochem. 2012;76(6):1068-74. doi: 10.1271/bbb.110666. Epub 2012 Jun 7. Biosci Biotechnol Biochem. 2012. PMID: 22790925
-
Effects of white rice, brown rice and germinated brown rice on antioxidant status of type 2 diabetic rats.Int J Mol Sci. 2012 Oct 10;13(10):12952-69. doi: 10.3390/ijms131012952. Int J Mol Sci. 2012. PMID: 23202932 Free PMC article.
-
Germinated brown rice ameliorates obesity in high-fat diet induced obese rats.BMC Complement Altern Med. 2016 May 23;16:140. doi: 10.1186/s12906-016-1116-y. BMC Complement Altern Med. 2016. PMID: 27216718 Free PMC article.
Cited by
-
Lipid Droplets Metabolism Mediated by ANXA7-PPARγ Signaling Axis Regulates Spinal Cord Injury Repair in Mice.Adv Sci (Weinh). 2025 Apr;12(16):e2417326. doi: 10.1002/advs.202417326. Epub 2025 Feb 25. Adv Sci (Weinh). 2025. PMID: 39996504 Free PMC article.
-
Nutrigenomic Effects of White Rice and Brown Rice on the Pathogenesis of Metabolic Disorders in a Fruit Fly Model.Molecules. 2023 Jan 5;28(2):532. doi: 10.3390/molecules28020532. Molecules. 2023. PMID: 36677591 Free PMC article.
References
-
- Hu P., Dharmayat K.I., Stevens C.A.T., Sharabiani M.T.A., Jones R.S., Watts G.F., Genest J., Ray K.K., Vallejo-Vaz A.J. Prevalence of familial hypercholesterolemia among the general population and patients with atherosclerotic cardiovascular disease: A systematic review and meta-analysis. Circulation. 2020;141:1742–1759. doi: 10.1161/CIRCULATIONAHA.119.044795. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

