The History of the Molybdenum Cofactor-A Personal View
- PMID: 35956883
- PMCID: PMC9370521
- DOI: 10.3390/molecules27154934
The History of the Molybdenum Cofactor-A Personal View
Abstract
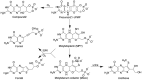
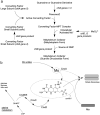
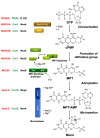
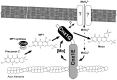
The transition element molybdenum (Mo) is an essential micronutrient for plants, animals, and microorganisms, where it forms part of the active center of Mo enzymes. To gain biological activity in the cell, Mo has to be complexed by a pterin scaffold to form the molybdenum cofactor (Moco). Mo enzymes and Moco are found in all kingdoms of life, where they perform vital transformations in the metabolism of nitrogen, sulfur, and carbon compounds. In this review, I recall the history of Moco in a personal view, starting with the genetics of Moco in the 1960s and 1970s, followed by Moco biochemistry and the description of its chemical structure in the 1980s. When I review the elucidation of Moco biosynthesis in the 1990s and the early 2000s, I do it mainly for eukaryotes, as I worked with plants, human cells, and filamentous fungi. Finally, I briefly touch upon human Moco deficiency and whether there is life without Moco.
Keywords: gephyrin; molybdenum; molybdenum cofactor biosynthesis; molybdopterin; nitrate reductase.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
-
- Pau R.N., Lawson D.M. Transport, homeostasis, regulation, and binding of molybdate and tungstate to proteins. Met. Ions Biol. Syst. 2002;39:31–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

