Recent Strategies in Transition-Metal-Catalyzed Sequential C-H Activation/Annulation for One-Step Construction of Functionalized Indazole Derivatives
- PMID: 35956893
- PMCID: PMC9370621
- DOI: 10.3390/molecules27154942
Recent Strategies in Transition-Metal-Catalyzed Sequential C-H Activation/Annulation for One-Step Construction of Functionalized Indazole Derivatives
Abstract
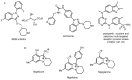
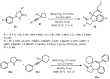
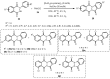
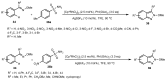
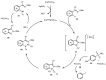
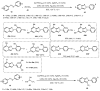
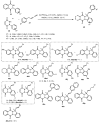
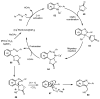
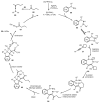
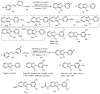
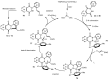
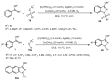
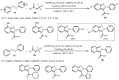
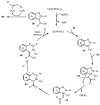
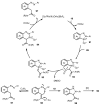
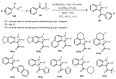
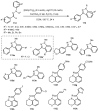
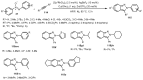
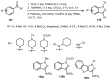
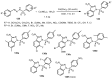
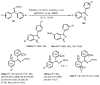
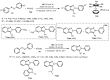
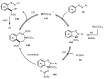
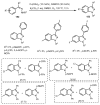
Designing new synthetic strategies for indazoles is a prominent topic in contemporary research. The transition-metal-catalyzed C-H activation/annulation sequence has arisen as a favorable tool to construct functionalized indazole derivatives with improved tolerance in medicinal applications, functional flexibility, and structural complexity. In the current review article, we aim to outline and summarize the most common synthetic protocols to use in the synthesis of target indazoles via a transition-metal-catalyzed C-H activation/annulation sequence for the one-step synthesis of functionalized indazole derivatives. We categorized the text according to the metal salts used in the reactions. Some metal salts were used as catalysts, and others may have been used as oxidants and/or for the activation of precatalysts. The roles of some metal salts in the corresponding reaction mechanisms have not been identified. It can be expected that the current synopsis will provide accessible practical guidance to colleagues interested in the subject.
Keywords: C–H functionalization; cyclization; indazole products; transition metals catalysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures























Similar articles
-
An Update of Transition Metal-Catalyzed Decarboxylative Transformations of Cyclic Carbonates and Carbamates.Molecules. 2019 Oct 31;24(21):3930. doi: 10.3390/molecules24213930. Molecules. 2019. PMID: 31683557 Free PMC article. Review.
-
Synthesis of indazoles and azaindazoles by intramolecular aerobic oxidative C-N coupling under transition-metal-free conditions.Chemistry. 2014 Apr 1;20(14):3932-8. doi: 10.1002/chem.201304923. Epub 2014 Mar 3. Chemistry. 2014. PMID: 24590598
-
Exploration of C-H Transformations of Aldehyde Hydrazones: Radical Strategies and Beyond.Acc Chem Res. 2018 Feb 20;51(2):484-495. doi: 10.1021/acs.accounts.7b00565. Epub 2018 Jan 23. Acc Chem Res. 2018. PMID: 29359909
-
Recent advances in the synthesis of ferrocene derivatives via 3d transition metal-catalyzed C-H functionalization.Org Biomol Chem. 2022 May 26;20(20):4061-4073. doi: 10.1039/d2ob00558a. Org Biomol Chem. 2022. PMID: 35521690 Review.
-
Transition Metal-Catalyzed Intermolecular Cascade C-H Activation/Annulation Processes for the Synthesis of Polycycles.Chemistry. 2021 Jan 4;27(1):121-144. doi: 10.1002/chem.202002110. Epub 2020 Oct 15. Chemistry. 2021. PMID: 32530508 Review.
Cited by
-
Recent Advances in Synthetic Strategies and Biological Properties of Indazole Scaffolds: A Review.Top Curr Chem (Cham). 2025 Jul 1;383(3):26. doi: 10.1007/s41061-025-00509-9. Top Curr Chem (Cham). 2025. PMID: 40591193 Free PMC article. Review.
References
-
- Shiri P. An Overview on The Copper-promoted Synthesis of Five-membered Heterocyclic Systems. Appl. Organomet. Chem. 2020;34:e5600. doi: 10.1002/aoc.5600. - DOI
-
- Alizadeh A., Roosta A., Rezaiyehraad R. Efficient Access to Pyrido[1, 2-a]pyrimidines and Imidazo[1, 2-a]pyridines Through Knoevenagel Reaction/aza–ene Addition/Intramolecular Cyclization. J. Iran. Chem. Soc. 2020;17:1123–1130. doi: 10.1007/s13738-019-01845-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

