Hybrid Molecules Containing Naphthoquinone and Quinolinedione Scaffolds as Antineoplastic Agents
- PMID: 35956896
- PMCID: PMC9370406
- DOI: 10.3390/molecules27154948
Hybrid Molecules Containing Naphthoquinone and Quinolinedione Scaffolds as Antineoplastic Agents
Abstract
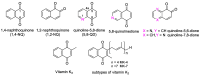
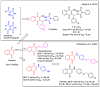
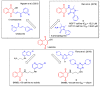
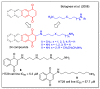
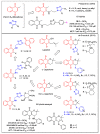
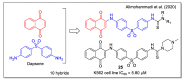
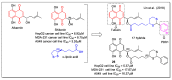
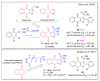
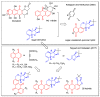
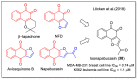
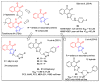
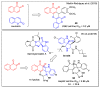
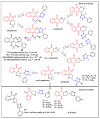
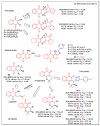
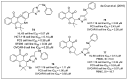
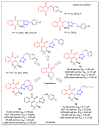
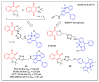
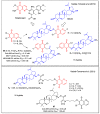
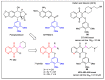
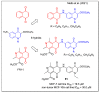
In recent decades, molecular hybridization has proven to be an efficient tool for obtaining new synthetic molecules to treat different diseases. Based on the core idea of covalently combining at least two pharmacophore fragments present in different drugs and/or bioactive molecules, the new hybrids have shown advantages when compared with the compounds of origin. Hybridization could be successfully applied to anticancer drug discovery, where efforts are underway to develop novel therapeutics which are safer and more effective than those currently in use. Molecules presenting naphthoquinone moieties are involved in redox processes and in other molecular mechanisms affecting cancer cells. Naphthoquinones have been shown to inhibit cancer cell growth and are considered privileged structures and useful templates in the design of hybrids. The present work aims at summarizing the current knowledge on antitumor hybrids built using 1,4- and 1,2-naphthoquinone (present in natural compounds as lawsone, napabucasin, plumbagin, lapachol, α-lapachone, and β -lapachone), and the related quinolone- and isoquinolinedione scaffolds reported in the literature up to 2021. In detail, the design and synthetic approaches adopted to produce the reported compounds are highlighted, the structural fragments considered in hybridization and their biological activities are described, and the structure-activity relationships and the computational analyses applied are underlined.
Keywords: anticancer; cytotoxicity; drug design; hybrid molecules; isoquinolinediones; medicinal chemistry; multitarget compounds; naphthoquinones; organic synthesis; quinolinequinones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures















References
-
- Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer; Lyon, France: 2020. [(accessed on 27 February 2022)]. Available online: https://gco.iarc.fr/today.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

